Translate this page into:
The effect of L-arginine on arterial stiffness and oxidative stress in chronic kidney disease
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chronic kidney disease (CKD) is a growing problem worldwide. The disproportionate increase in the burden of cardiovascular disease in patients with CKD may be significantly contributed by nontraditional risk factors. Increased arterial stiffness has been recognized as an important player in contributing to this morbidity and mortality. The aim of this study was to report the effect of L-arginine on arterial stiffness and oxidative stress in patients with CKD. Thirty patients with stage II to IV CKD were administered 9 g of L- arginine per day orally for a period of 12 weeks. The parameters evaluated at baseline, at 8 weeks, and at the end of 12 weeks were serum nitric oxide (NO), carotid.femoral pulse wave velocity (cf PWV), and radial artery pulse wave analysis which included aortic augmentation pressure (AP), aortic augmentation index (AIx), aortic augmentation index at heart rate of 75 bpm, subendocardial viability ratio, radial pressures, and central aortic pressure. Serum levels of NO and malondialdehyde (MDA) were estimated at baseline and at the end of 12 weeks. The control group was composed of age- and sex-matched healthy individuals. Twenty-five patients completed the study. Two patients were lost to follow.up; three patients developed adverse events and were excluded. Baseline NO levels were low (13.55 ± 7.49 μM/L) in all the subjects. Administration of L-arginine resulted in improvement in the carotid-radial PWV (m/s) (10.08 ± 1.72 at baseline to 8.56 ± 1.16 by 12 weeks; P < 0.001), cf PWV (m/s) (13.06 ± 2.65 at baseline to 10.62 ± 1.93 at 12 weeks; P < 0.001), Aortic Augmentation Index (%) (32 ± 10.34 at baseline to 17.84 ± 8.05 at 12 weeks; P < 0.001), aortic augmentation pressure (mm of Hg) (14.03 ± 6.53 at baseline to 7.12 ± 3.85 at 12 weeks; P < 0.001), and NO (μM/L) (13.55 ± 7.49 at baseline to 30.22 ± 9.8 at 12 weeks; P < 0.001). There was no significant change in the levels of MDA (nanomol/ml) (20.0 ± 10.14 at baseline and 19.16 ± 9.36 at 12 weeks; P = ns). In conclusion, PWV, an indicator of arterial stiffness, is greatly increased even in the early stages of CKD. Supplementation of L-arginine is a safe, well-tolerated, and effective way of improving endothelial dysfunction in patients with CKD.
Keywords
Arterial stiffness
chronic kidney disease
L-arginine
oxidative stress
pulse wave velocity
Introduction
Chronic kidney disease (CKD) is a growing problem worldwide. Cardiovascular disease (CVD) and accounts for about 50% of deaths in these patients.[1–3] This disproportionate increase in the burden of CVD may be significantly contributed by nontraditional risk factors.
Increased arterial stiffness has been recognized as an important player contributing to this morbidity and mortality.
Mechanisms implicated for increased stiffness in patients with uremia include chronic fluid overload, arterial calcifications, microinflammation, sympathetic nervous system overactivity, activation of the renin-angiotensin-aldosterone system, increased lipid oxidation, and abnormalities in the nitric oxide (NO) system. The result is the diffuse process of arteriosclerosis, characterized by stiffer arteries due to reduced arterial elasticity or compliance. These lead to a higher systolic blood pressure, lower diastolic blood pressure, and widened PP which are known major determinants for high CV morbidity and mortality.
The measurement of pulse wave velocity (PWV) is generally accepted as the most simple, noninvasive, robust, and reproducible method to determine arterial stiffness. Carotid-femoral PWV (cf PWV), considered as the “gold-standard” measurement of arterial stiffness, is a direct measurement, and it corresponds to the widely accepted propagative model of the arterial system.[4]
Endothelial dysfunction is an early marker for atherosclerosis and makes endothelium a logical target for prevention of cardiovascular morbidity and mortality. Despite its important role in a wide range of homeostatic processes and its potential as a therapeutic target, the endothelium continues to be overlooked in clinical practice. One reason for the bench to bedside gap may be the inaccessibility of the endothelium in the patient.
The intravenous or intracoronary administration of L-arginine, the physiologic precursor for NO, can acutely improve endothelium-dependent, but not endothelium-independent vasodilation in patients with hypercholesterolemia or coronary atherosclerosis. Beneficial effects of L-arginine supplementation have been reported in several models of CKD including renal ablation, ureteral obstruction, puromycin-induced nephrosis and nephropathy secondary to diabetes, salt-sensitive hypertension, radio-contrast agents, and aging.[5–10] In this article, we report the effect of L-arginine on arterial stiffness and oxidative stress in patients with CKD.
Materials and Methods
This was an open-label study approved by the Institutional Ethical Committee. All subjects were included after obtaining the informed consent. Total duration of the study was 17 weeks which included a 1-week run-in period and 4 weeks of follow-up. The drugs were administered for 12 weeks.
Both male and female patients between the age group of 18 and 70 years, with an estimated glomerular filtration rate <90 ml/min for more than 3 months were included. The patients also needed to have a cf PWV ≥900 cm/s. A written informed consent was obtained from each patient.
Patients who had hypersensitivity to study drugs were excluded from the study. Similarly, renal transplant recipients, patients with end-stage renal disease on maintenance dialysis, pregnant and lactating women, patients with a serum albumin <3 g/dl, bilirubin more than 1.5 times the upper limit of normal (ULN), or transaminases more than 2.5 times the ULN were also excluded. Others who excluded were those receiving experimental therapies, those with previous or present malignancies, uncontrolled infection, coronary disease, and medically unstable conditions. Patients who were not willing to give written informed consent were also excluded from the study.
The control group was composed of healthy individuals who were age- and sex-matched with the study subjects in a 1:1 ratio. The control group was analyzed for baseline parameters only at the beginning of the study.
Parameters
The parameters evaluated at baseline, at 8 weeks, and at the end of 12 weeks were serum NO, serum MDA, cf PWV, and radial artery pulse wave analysis (PWA) which included aortic augmentation pressure (mm of Hg) (AP), aortic augmentation index (%) (AIx), AIx at heart rate of 75 bpm, subendocardial viability ratio (%), radial pressures, carotid-radial pulse wave velocity (cr PWV), and central aortic pressure.
Drug administration
Patients were instructed to take a 3-g sachet of L-arginine granules in 250 ml of water three times a day.
Visit I (-1 to 0 week)
Patients were informed in detail about the purpose, the protocol, and the procedures involved in the study, and a written informed consent was obtained. Details of demography were noted and physical examination was done. Ten milliliters of blood was collected, serum separated, and stored at -20°C for biochemical analysis. Radial PWA, cf PWV, and cr PWV were recorded. They were instructed to consume all the pre-existing medications if any, for the length of the study and return after 1 week.
Visit II (day 0)
Radial PWA, cf PWV, and cr PWV were recorded. L-arginine sachets were provided with written instructions (as stated above in “Drug administration”). Phone numbers were provided and the patients were asked to report if any adverse events occurred after the intake of the drug. Patients were instructed to come after 8 weeks.
Visit III (0 + 8 week)
Compliance was assessed by sachet count. The patients who had missed out 20% of the total study medication was considered noncompliant. Patients were enquired about the incidence of any adverse events during the past 8 weeks while on medication. Physical examination was done. Radial PWA, cf PWV, and cr PWV were recorded. L-arginine sachets were provided for another 4 weeks. They were advised to report if any adverse events occurred and were instructed to come after 4 weeks.
Visit IV (0+ 12 week)
Patients were informed about the completion of study. Compliance was assessed by sachet count, physical examination, and PWA as mentioned earlier.
Visit V (0 +16 week)
Physical, biochemical, and hematological evaluations were repeated and enquiry about the incidence of any adverse events while on medication during the last 8 weeks was made.
PWA and PWV
The procedure was performed in a quite temperature-controlled room (23°C) using SphygmoCor® system (Sphygmocor Pulse Wave Velocit System Model Scor Model, version 8; AtCor Medicals Pvt. Ltd, Sydney, Australia) with PWA and PWV options installed.
Statistical analysis
The statistical analysis was carried out using Sigma GraphPad Software, version 4. The values were expressed as mean ± SD. P < 0.05 was considered statistically significant. The intention to treat analysis was used and the differences in the characteristics of subjects at study entry and at 12 weeks after treatment with L-arginine were compared using the paired Student's t-test.
Results
Thirty patients were inducted into the study. Of these, 25 could complete the study. Two patients were lost to follow-up, and three patients developed adverse events. The mean age of the patients was 49.44 ± 11.08 (range 22-68) years. Patients comprised 80% males and 20% females. Seventeen patients were in CKD stage 3, five patients were in CKD stage 4, and three patients were in CKD stage 2. All the patients included were hypertensive and 12 patients had type 2 diabetes mellitus. The causes of CKD in other patients were IgA nephropathy in four patients, chronic glomerulonephritis in five patients, chronic interstitial nephritis in two patients, and obstructive uropathy in two patients. Eight patients were smokers and five of them were alcoholics too.
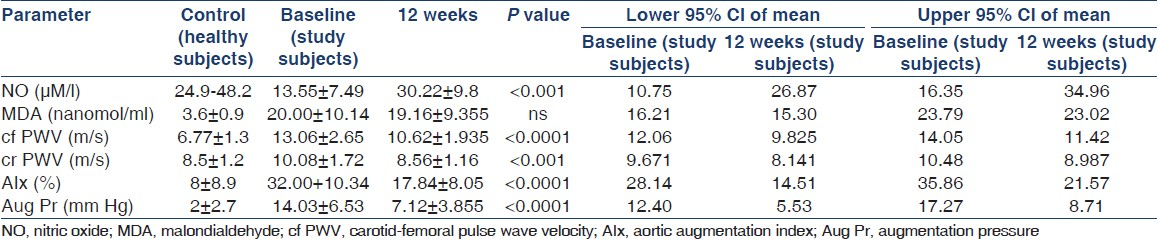
Baseline NO levels were lower (13.55 ± 7.49 μM/l) in all the subjects when compared with healthy controls [Table 1, Figure 1]. These levels were significantly worse at baseline in diabetics than nondiabetics [P = 0.001; Table 2]. Response to L-arginine was identical in both diabetics and nondiabetics and NO levels increased significantly (30.22 ± 9.8 μM/l; P < 0.001). The cf PWV, cr PWV, Aix, and augmentation pressure improved significantly with L-arginine [Table 1, Figures 2–5]. The baseline subendocardial viability ratio was 136.60 ± 25.36 and improved to 149.90 ± 21.83 (P < 0.002) by 12 weeks [Figure 6].

- Nitric oxide levels at 3 months after intervention


- Carotid radial PWV at 3 months after intervention

- Aortic augmentation index at 3 months after intervention

- Carotid-femoral pulse wave velocity at 3 months after intervention

- Augmentation pressure at 3 months after intervention

- Subendocardial viability ratio at 3 months after intervention
The systolic, diastolic, mean, and pulse pressure of aorta and brachial artery also showed a significant decline with L-arginine [Table 3] necessitating a reduction in the dose of antihypertensive drugs in 19 of the 25 patients during the study period.

The PWA and serum levels of NO and MDA reached baseline levels when evaluated at 16 weeks (i.e., 4 weeks after L-arginine administration was discontinued [not shown]). The other biochemical parameters evaluated at presentation and at the end of 12 weeks of study drug administration are as shown in Table 4.

Adverse events
Three patients were excluded due to adverse events. All of them developed diarrhea on the second day of administration of the study drug.
Discussion
The mechanisms linking CKD and CVD are not fully elucidated. The literature supports higher prevalence of arterial stiffness in patients with CKD.[11–13] Administration of L-arginine improves endothelium-dependent vasodilatation. Experimental and human studies have shown both therapeutic and detrimental effects of modification of L-arginine intake.[614–16]
Endothelial function is an important determinant of central hemodynamics and large artery stiffness. Among several ways to assess the arterial stiffness, the two most common methods used by virtue of their ease of reproducibility and simplicity are the PWV and AIx.[1718] PWV is greater in stiffer arteries and is related to increased mortality. Aortic PWV, as a measure of arterial stiffness, has proved useful in predicting cardiovascular morbidity and mortality in several populations of patients, including the healthy elderly hypertensive patients,[19] and those with end-stage renal disease receiving hemodialysis.[20] There are little data characterizing the aortic PWV in predialysis population. In our subjects, both the radial and aortic pressures were greater than that of the non-CKD population. Similar findings were also obtained by studies from Townsend et al.,[21] Shinohara et al.,[22] and Wang et al.[23] We could demonstrate the decrease in systolic, diastolic, mean, and pulse pressures of both the radial and aortic arteries following L-arginine administration.
cf PWV depends on the wall thickness and elasticity of the central aorta and ilio femoral arteries and is regarded as the most important measure of central arterial stiffness. The mean cf PWV and the mean crPWV were 13.06 and 10.08 m/sec, respectively. The velocity decreased significantly to 10.62 and 8.56 m/sec, respectively, with L-arginine. Although the cf PWV at baseline was significantly worse in the diabetics than in nondiabetics (13.77 ± 3.85 m/sec vs 10.69 ± 2.86 m/sec; P = 0.04), it decreased to a similar extent (P = 0.88) in both the participants. Hence, the drug was effective in both the subgroups.
The AIx is also associated with survival and it is a composite measure of the reflective properties of peripheral arteries and the elastic property of the large arteries.[4] All the participants had a decline in the AIx and augmentation pressure. It has been shown that myocardial ischemia is predisposed by increased aortic stiffness. This results from an increase in the Tension Time Index and a decrease in the aortic pressure during the diastole. The tonometric subendocardial viability ratio (SEVR) is a noninvasive estimate of myocardial perfusion relative to cardiac workload. SEVR less than 1 has been shown to signify subendocardial underperfusion mainly due to reduction in diastolic perfusion times. The SEVR improved with L-arginine as shown in Figure 6.
NO, a potent anti-atherogenic molecule, is a surrogate marker of risk for endothelial function and predicts all-cause cardiovascular mortality. There can be several attributes for the decreased availability of NO in patients with CKD. Decreased availability of L-arginine, a substrate for NO synthesis, decreased renal biosynthesis of L-arginine, and increased circulating levels of ADMA, an endogenous inhibitor of NOS are some of them. Several interventions were tried to reverse endothelial dysfunction and one of them was to increase the NO production for which L-arginine could be a valuable tool.
The data on the effects of L-arginine in humans are conflicting. Experimental studies in animal models had shown an increase in the plasma levels of NO with L-arginine therapy[24] but were challenged in the more recent studies in humans. In a study involving patients with type 1 diabetes, Mullen et al. did not find an improvement in endothelial function with L-arginine.[25] In a randomized trial involving 30 patients with coronary artery disease, Blum et al. found that 1-month therapy with L-arginine (9 g a day) had no effect on the NO bioavailability although the plasma levels of arginine had shown an increase.[26] In another randomized trial involving 25 normotensive children with CKD, Kathy et al. showed similar findings of an increase in plasma arginine concentration but with no improvement in endothelial dysfunction.[27] In contrast, a clinical benefit with L-arginine was shown by Maxwell et al. in a randomized study of 36 patients with angina.[28]
The mean level of NO at the start of our study was lower than the levels seen in healthy population [Table 1]. Plasma levels of NO in CKD patients with type 2 diabetes mellitus were lower than in patients without diabetes [Table 2]. We could show a significant increase in the plasma levels of NO from a mean of 13.55 μM/L ± 7.49 to 30.22 μM/L ± 9.8 (P < 0.001) with L-arginine therapy [Table 1], and this effect was significant both in patients with or without diabetes [Table 2]. This increase in the plasma NO levels may help to retard the progression of CKD as shown in experimental studies in mice.[6]
MDA is a secondary product of oxidative stress formed during lipid peroxidation (LPO) and is measured using the colorimetric assay of thiobarbituric acid. MDA being a reactive electrophile species can form covalent protein adducts which are referred to as advanced lipoxidation end products. The measurement of MDA is one of the frequent ways adopted to estimate the levels of LPO. Studies in mice subjected to ischemia reperfusion injury had shown an increase in the level of MDA following pretreatment with L-arginine[29] which was detrimental. The MDA levels in our study showed a marginal decline and this was not statistically significant. This reinforces the fact that the beneficial effects of L-arginine are NO-dependent and has little role as an antioxidant. L-arginine did not show any influence on the renal function tests, liver function tests, or lipid profile as shown in Table 4.
There are some limitations in our study. First, smoking by itself causes a change in endothelial function. Our study included smokers and this could have contributed to the endothelial dysfunction at the baseline and there were no smokers in the control group. It would have been ideal to exclude smokers from the study. Second, our study population was small; a much larger randomized controlled trial would be necessary to establish the results obtained in this study. Measurement of change in the diameter of brachial artery was not carried out and this could be included in further studies.
In summary, we could show that PWV, an indicator of arterial stiffness, was increased even in the early stages of CKD. Supplementation of L-arginine can be a safe, tolerable, and an effective way of improving endothelial dysfunction and NO production and may have a good therapeutic potential in reducing cardiovascular morbidity and mortality in patients with CKD.
Source of Support: Nil
Conflict of Interest: L-arginine was provided by Ms Sai Mirra Innopharm Pvt Ltd, SIDCO estate, Chennai.
References
- The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310-4.
- [Google Scholar]
- Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112-S9.
- [Google Scholar]
- Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-305.
- [Google Scholar]
- Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588-605.
- [Google Scholar]
- Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int. 1997;52:171-81.
- [Google Scholar]
- Dietary supplementation with L-arginine ameliorates the progression of renal disease in rats with subtotal nephrectomy. Am J Kidney Dis. 1992;20:168-76.
- [Google Scholar]
- Oral administration of L-arginine and captopril in rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995;47:1515-21.
- [Google Scholar]
- EDRF role in renal function and blood pressure of normal rats and rats with obstructive uropathy. Kidney Int. 1992;41:403-13.
- [Google Scholar]
- In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945-51.
- [Google Scholar]
- Long-term administration of L-arginine improves nitric oxide release from kidney in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1994;23:752-6.
- [Google Scholar]
- Arterial stiffness in predialysis patients with uremia. Kidney Int. 2004;65:936-43.
- [Google Scholar]
- Hemodialysis acutely improves endothelium-independent vasomotor function without significantly influencing the endothelium-mediated abnormal response to a beta 2-agonist. Nephrol Dial Transplant. 2004;19:637-43.
- [Google Scholar]
- Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202-7.
- [Google Scholar]
- L-arginine supplementation accelerates renal fibrosis and shortens life span in experimental lupus nephritis. Kidney Int. 2003;63:1382-92.
- [Google Scholar]
- From rats to man: a perspective on dietary L-arginine supplementation in human renal disease. Nephrol Dial Transplant. 1999;14:1640-50.
- [Google Scholar]
- Dietary supplementation with L-arginine ameliorates glomerular hypertension in rats with subtotal nephrectomy. J Am Soc Nephrol. 1994;4:1690-4.
- [Google Scholar]
- Reproducibility of derived central arterial waveforms in patients with chronic renal failure. Clin Sci (Lond). 2002;103:59-65.
- [Google Scholar]
- Assessment of arterial distensibility by automatic pulse wave velocity measurement.Validation and clinical application studies. Hypertension. 1995;26:485-90.
- [Google Scholar]
- Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184-9.
- [Google Scholar]
- Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852-60.
- [Google Scholar]
- Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282-9.
- [Google Scholar]
- Arterial stiffness in predialysis patients with uremia. Kidney Int. 2004;65:936-43.
- [Google Scholar]
- Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494-501.
- [Google Scholar]
- Borders, Tandem antifibrotic actions of L-arginine supplementation and low protein diet during the repair phase of experimental glomerulonephritis. Kidney Int. 2000;57:992-1001.
- [Google Scholar]
- Atorvastatin but not L-arginine improves endothelial function in type 1 diabetes: a double-blind study. J Am Coll Cardiol. 2000;36:410-6.
- [Google Scholar]
- Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000;101:2160-4.
- [Google Scholar]
- Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int. 2002;62:1372-8.
- [Google Scholar]
- Randomized trial of medical food for dietary management of chronic, stable angina. J Am Coll Cardiol. 2002;39:37-45.
- [Google Scholar]
- Effects of L-arginine on the kidney levels of malondialdehyde in rats submitted to renal ischaemia-reperfusion. BJU Int. 2001;88:273-7.
- [Google Scholar]







