Translate this page into:
Non-O1, non-O139 Vibrio cholerae sepsis in a patient with nephrotic syndrome
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Non-O1, non-O139 Vibrio cholerae is an encapsulated bacterium, ubiquitous in the marine environment and generally considered to be non-pathogenic. However, it is known to cause diarrheal illness, wound infection, and bacteremia in immunocompromised hosts. Here we have describe non-O1, non-O139 V. cholerae sepsis in a patient with nephrotic syndrome following exposure to sea-water. Interestingly, the exposure occurred remotely 4 months prior to the onset of nephrotic syndrome. The occurrence of florid sepsis after a prolonged interval from the time of exposure is peculiar and raises the possibility of an association between occult Vibrio sepsis and nephrotic syndrome.
Keywords
Immunocompromised
nephrotic syndrome
non-O1
non-O139
sepsis
vibrio cholera
Introduction
Vibrio cholerae is a gram-negative, motile, comma-shaped bacterium. More than 200 serotypes have been identified based on the lipopolysaccharide O antigen. Of these, O1 and O139 serotypes are associated with diarrheal illness. Non-O1, non-O139 V. cholerae strains are generally considered to be non-pathogenic. However, intestinal and extra-intestinal infections with non-O1, non-O139 strains have occurred both sporadically as well as in outbreaks.[12] There are several reports of such strains causing sepsis in immunocompromised hosts such as those with liver disease and hematological disorders.[3–6] The occurrence of non-O1, non-O139 V. cholerae infection in nephrotic syndrome is quite rare and has been reported only once previously.[7]
Case Report
A 33-year-old lady presented with a history of recurrent episodes of headache, myalgia, and occasional episodes of nausea and vomiting for 5 months duration. She was evaluated elsewhere and was treated for migraine, without relief. She had intermittent episodes of hypotension with persistent leucocytosis, for which she was treated with multiple courses of empirical broad-spectrum antibiotics. She developed anasarca 1 month prior to visiting our centre. There was no history of hematuria or reduction in urine output.
On examination, she was afebrile; blood pressure (BP) was 110/70 mmHg without postural hypotension. Systemic examination was unremarkable. Investigations revealed nephrotic range proteinuria (14.3 g/day), microscopic hematuria (10-12 red blood corpuscle/high power field), hypoalbuminemia (0.8 g/dl), hypercholesterolemia (455 mg/dl), hypertriglyceridemia (352 mg/dl), and a serum creatinine of 1.08 mg/dl. She also had leucocytosis (15,500 cells/mm3) with a differential count of neutrophil 71%, lymphocyte 19%, eosinophil 3%, monocyte 6%, and basophil 1%. Anti-nuclear antibody (ANA) and anti-double stranded deoxyribonucleic acid antibody (anti-dsDNA) were negative; Complement C3c and C4 levels were normal.
She was started on diuretics and intravenous albumin for edema. Blood culture, performed in view of leucocytosis at admission, did not grow any pathogen. Renal biopsy revealed six glomeruli displaying mild increase in mesangial matrix with hypercellularity [Figure 1]. Immunofluorescence study was negative. A diagnosis of idiopathic unsampled focal and segmental glomerulosclerosis was made and she was started on prednisolone at a dose of 120 mg every alternate day. Subsequently, electron microscopy revealed mesangial proliferative glomerulonephritis with few small mesangial electron dense deposits. She was discharged to follow-up as an out-patient.
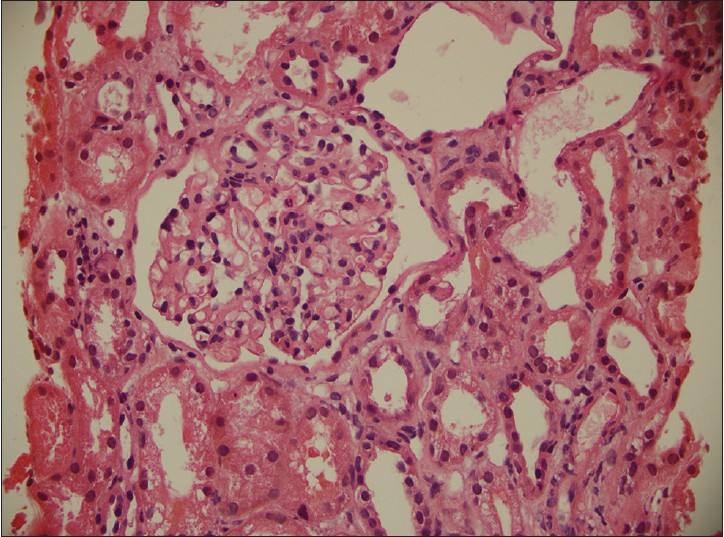
- There is mild increase in mesangial matrix with mesangial hypercellularity, mild thickening of the paramesangial capillary walls with narrowing of capillary lumina. The extraglomerular compartment showed focal mild interstitial fibrosis, focal tubular dilatation with flattening of lining epithelium, hyaline casts, focal infiltrates of lymphocytes and occasional eosinophils
Five days after starting oral steroids, she presented to the emergency department with myalgia, giddiness, and weakness. There was no history of fever. On examination, she had tachycardia, tachypnea, and hypotension. Systemic examination was unremarkable. Investigations revealed worsening leucocytosis (15,900 cells/mm3) with neutrophilic predominance (89%) and markedly elevated serum procalcitonin levels (144.5 ng/ml), suggesting possible bacterial sepsis with septic shock. After drawing blood for culture, she was started on broad-spectrum antibiotic coverage with intravenous meropenem. The total leucocyte count increased to 53,500 cells/mm3 the next day. Imaging of chest and abdomen did not reveal any focus of sepsis. The patient was managed in the intensive care unit with meropenem and inotropic support. Clinical improvement started after 72 hours of antibiotics with reduction of inotropic requirement and normalization of the total leucocyte count by the fourth day. Her blood culture grew non-O1, non-O139 V. cholerae, which was sensitive to ampicillin, tetracycline, cotrimoxazole, cefotaxime, ofloxacin, and meropenem. In view of V. cholerae sepsis, a stool culture was done, which did not grow any pathogen.
On further questioning, she recalled that 16 weeks before the onset of nephrotic syndrome, she had visited a beach for swimming and bathing. She did not consume any sea-food. The patient completed 2 weeks of meropenem, following which a repeat blood culture was documented to be sterile. She was then discharged in stable condition with advice to continue prednisolone for nephrotic syndrome.
Discussion
Non-O1, non-O139 V. cholerae is an encapsulated bacterium, ubiquitous in the marine environment and known to cause diarrheal illness, wound infection, and bacteremia in immunocompromised hosts.[6] Infections have been described in patients with liver disease and hematological disorders.[3–6] Our case illustrates a rare instance of non-O1, non-O139 V. cholerae sepsis occurring in a patient with nephrotic syndrome. To our knowledge, only one such case has been reported in nephrotic syndrome so far.[7]
In our patient, the exposure to sea-water occurred 4 months prior to the onset of nephrotic syndrome. Shelton et al.,[3] reported occurrence of two episodes of non-O1 Vibrio bacteremia in a patient with multiple myeloma at 11 months interval with sea-water exposure occurring only before the first episode. In our patient, throughout her illness, she had received intermittent courses of antibiotics for unexplained leucocytosis and hypotension, which had probably prevented full-blown sepsis from manifesting earlier, but failed to eradicate the organism. In our patient, the exposure was limited to swimming and bathing, with no consumption of sea-food. There are similar reports of patients developing bacteremia after exposure to just sea-water with no consumption of sea-food.[36]
Nephrotic syndrome is an immunocompromised state with dysfunctional T cells, loss of protective immunoglobulins, and decreased levels of complement pathway factors.[8–10] Patients with nephrotic syndrome are at increased risk of developing infections with encapsulated bacteria due to the loss of opsonizing factors in urine.[8] Some strains of non-O1, non-O139 V. cholerae have been shown to be encapsulated that may enhance the ability of this organism to cause sepsis in patients with nephrotic syndrome.[11] In addition, the treatment of nephrotic syndrome with steroids could have worsened the patient’s immune status further, predisposing her to fulminant sepsis with septic shock.
On the other hand, it is reasonable to consider whether the smoldering Vibrio infection precipitated the nephrotic syndrome in this patient. Infections are known to be associated with glomerular pathology like mesangial-proliferative glomerulonephritis. Mesangial cells can be stimulated by lipopolysaccharides and inflammatory cytokines to induce mesangial cell proliferation and extracellular matrix production, which is the hallmark of mesangioproliferative glomerulonephritis. Nevertheless, the occurrence of such involvement secondary to Vibrio infection has not been reported previously. The temporal profile of exposure to seawater, features of smoldering sepsis, followed by the onset of nephrotic syndrome and the presence of few mesangial electrone dense deposits with lack of frank features of podocytopathy raises the speculation of an infection-associated glomerulopathy, indicating a role of Vibrio infection in precipitating the nephrotic syndrome.
To conclude, this report illustrates a rare occurrence of non-O1, non-O139 V. cholerae sepsis in a patient with nephrotic syndrome. The occurrence of florid sepsis after a prolonged interval from the time of exposure it raises the possibility of an association between occult Vibrio sepsis and nephrotic syndrome.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Clinical and environmental isolates of Vibrio cholerae O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J Clin Microbiol. 2001;39:4086-92.
- [Google Scholar]
- Epidemic of diarrhea caused by Vibrio cholerae non-O1 that produced heat stable toxin among Khmers in a camp in Thailand. J Clin Microbiol. 1993;31:1315-7.
- [Google Scholar]
- Recurrent non-O: 1 Vibrio cholerae bacteremia in a patient with multiple myeloma. Cancer. 1993;72:105-7.
- [Google Scholar]
- Non-O1, non-O139 Vibrio cholerae bacteremia in a cirrhotic patient. J Med Microbiol. 2010;59:1260-2.
- [Google Scholar]
- Vibrio cholerae (non-O1, non-O139) sepsis in a child with Fanconi anemia. Diagn Microbiol Inf Dis. 2004;50:287-9.
- [Google Scholar]
- Infections due to non-O1 Vibrio cholerae in Southern Taiwan: Predominance in cirrhotic patients. Clin Infect Dis. 1998;27:774-80.
- [Google Scholar]
- Non-O1 Vibrio cholerae bacteremia and peritonitis in a patient with nephrotic syndrome. Pediatr Infect Dis J. 1996;15:276-7.
- [Google Scholar]
- Decreased serum factor B concentration associated with decreased opsonization of Escherichia coli in the idiopathic nephrotic syndrome. Pediatr Res. 1977;11:910-6.
- [Google Scholar]
- Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal-change nephrotic syndrome. N Engl J Med. 1975;293:8-12.
- [Google Scholar]
- T-Cell dysfunction in minimal-change nephrotic syndrome of childhood. Am J Dis Child. 1982;136:713-7.
- [Google Scholar]
- Non-O1 Vibrio cholerae NRT36S produces a polysaccharide capsule that determines colony morphology, serum resistance, and virulence in mice. Infect Immun. 1992;60:864-9.
- [Google Scholar]







