Translate this page into:
Renal histology in diabetic nephropathy: A novel perspective
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease all over the world. India has a high incidence and prevalence of diabetes and >30% have nephropathy. Recently, a histological classification has been proposed. This study analyzed the renal histology in 114 diabetic patients with renal dysfunction. Nearly 75% of patients had DN. Fifty five (63.95%) were males. Mean duration of diabetes was 7.04 ± 4.9 years. Mean serum creatinine in study group was 5.2 ± 2.9 mg/dl, with mean estimated glomerular filtration rate of 23.43 ± 21.48 ml/min/1.732 m2. Forty eight patients (55.81%) had diabetic retinopathy (DR); prevalence of DR was more in patients who had diabetes for > 10 years than patients who had diabetes for <6 years (P = 0.022). The most common histological class was Class IV observed in 37 (43.02. %) cases, Class III DN in 24 (27.90%) cases, Class IIa and Class IIb in 11 (12.79%) cases each and Class I DN in 3 (3.48%) cases. Higher histological class was associated with higher proteinuria, lower glomerular filtration rate (P < 0.001) and was more likely to be associated with retinopathy (P = 0.012) and hypertension (P = 0.0003) but did not correlate with duration of diabetes (P = 0.85). There was a poor correlation between retinopathy and DN. Biopsy helps to stage the renal lesions in diabetics with renal dysfunction.
Keywords
Diabetes
diabetic nephropathy
non-diabetic renal disease
renal histology
retinopathy
Introduction
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease (ESRD) around the globe.[1] Projections from the recent Indian Council of Medical Research–India Diabetes study has shown that India has 62.4 million people with diabetes making DN an important cause of renal failure.[2] Asia has a high prevalence of DN.[3] A study from India has shown that 31.3% of renal failure in India is caused by DN.[4] Recently a histological classification was proposed for DN.[5] This study was carried out to determine the histopathological lesions in DN and to correlate clinical findings with histology.
Aim of the study
This study was carried out to determine various renal histopathological lesions in diabetic patients with renal dysfunction and to establish clinic-pathological correlation.
Materials and Methods
All diabetic patients (type I and II) admitted in the wards of nephrology, Osmania General Hospital, Hyderabad with renal dysfunction (either proteinuria >3000 mg or elevated serum creatinine) were included in the study. Patients with acute precipitating factors for renal dysfunction, isolated micro-albuminuria, those with bilateral contracted kidneys, patients with contraindications for renal biopsy or those unwilling for renal biopsy were excluded.
The prospective study was carried out from November 2010 to August 2013 in Department of Nephrology, Osmania General Hospital. Of 302 diabetic patients with renal dysfunction, 188 had contracted kidneys, bleeding diathesis or were unwilling for biopsy and hence excluded from the study. Remaining 114 patients were subjected to renal biopsy. The study protocol was approved by the Human Ethics Review Committee of Osmania Medical College and an informed consent was obtained from each patient. Diagnosis of diabetes was made using American Diabetes Association criteria for diagnosis of diabetes.[6] Detailed analysis with respect to history, duration of diabetes, mode of treatment for diabetes, renal symptoms, details of micro- and macro-vascular complications of diabetes was done. Each patient underwent a comprehensive clinical examination. Fundus examination was performed by single ophthalmologist. Fluorescein angiography was done when indicated. Investigations included renal profile (blood urea, serum creatinine, serum electrolytes, complete urine examination and for 24 h proteinuria estimation). Glomerular filtration rate (GFR) was calculated by the Cockcroft-Gault formula in adults and Schwartz formula for children. Random, fasting and post-prandial blood sugar, complete hemogram, liver function tests, clotting time, bleeding time, prothrombin time, international normalized ratio and appropriate imaging and radiological investigations were done. Ultrasonographic examination was carried out to assess the renal size. Renal biopsy was performed in 114 patients after stabilization under ultrasound guidance with a biopsy gun (BARD gun 16/18 G, 22 mm, cutting edge). Three samples were collected in all; samples were analyzed under light microscope (LM) and immunofluorescence (IF) by a single pathologist. Electron microscopy was also done if classification was not possible by LM or IF. All universal precautions were executed during the biopsy. Processed tissue was stained with hematoxylin and eosin, periodic acid Schiff, silver methenamine and Masson trichrome. Immunofluorescence microscopy was done after staining with fluorescent labeled antisera to IgG, IgM, IgA, C3, CIq and fibrinogen. The intensity was semi-quantitatively scored, as 0 for negative, 1 + for present, 2 + for definite and 3 + for strongly positive. Renal lesions in DN were classified according to “pathologic classification of DN” by Tervaert et al. Renal Pathology Society (RPS).[5] This classification scheme is based on glomerular lesions because these are relatively easy to recognize with good inter observer agreement and because glomerular lesions best reflect the natural course of progressive DN. Of course, glomerular and interstitial lesions contribute to the decline in renal function in DN and may be independent factors in the progression of DN; however, many studies also show that severity of chronic interstitial and glomerular lesions are closely associated. In the present study of type 1 and type 2 diabetic cases are classified together as suggested in RPS classification and clinicopathological correlation was analyzed.
Histopathology was analyzed in correlation with age, sex, duration of diabetes mellitus (DM), proteinuria, retinal finding on fundus examination, renal function tests/GFR, associated hypertension, glycemic control and need for renal replacement therapy. Statistical analysis was performed by utilizing IBM SPSS Inc 19 software at department of biostatistics, National Institute of nutrition, Hyderabad. Frequency tables were made to estimate the frequency and percentage of each parameter analyzed. Descriptive statistics were expressed in terms of minimum, maximum, mean and standard deviation. Logistic regression was used for the prediction of occurrence of an event. Categorical data were compared using Chi-square test and means were compared using Student's t-test (for two groups) or by analysis of variance (for more than two groups). P < 0.05 was considered to be significant.
Results
A total of 114 diabetic patients with renal dysfunction (defined as nephritic proteinuria or elevated serum creatinine) underwent renal biopsy. None of the patients developed macroscopic hematuria or needed hospitalization. 2 patients had post biopsy pain which subsided with analgesics. Eighty six (75.43%) patients had DN on biopsy. Twenty eight patients had non-diabetic renal disease (NDRD) either alone or superimposed on DN and were not analyzed in this study. Out of 86 patients type 1 diabetes was present in 8 cases and 78 cases had type 2 diabetes.
Fifty five (63.95%) patients were male and 31 (36.09%) were females with a male: female ratio of 1.7:1. Mean age was 50 ± 13 years (range 6-75 years). Sixty five (75.58%) patients were between 30 and 60 years of age while 6 (6.9%) were <30 years and 15 (17.4%) were >60 years of age.
In these 86 patients mean duration of diabetes was 7.04 ± 4.9 years. Thirty two (37.2%) had diabetes <6 years duration, 32 (37.2%) had between 6 and 10 years duration while 22 (25.58%) had diabetes for >10 years.
Mean 24 h urine protein in the study group was 3.68 ± 2.44 g/day. Forty seven patients (54.65%) had subnephrotic proteinuria and 12 patients (13.95%) had proteinuria of >5 g/day.
Mean serum creatinine in study group was 5.2 ± 2.9 mg/dl, with mean estimated glomerular filtration rate (eGFR) 23.43 ± 21.48 ml/min/1.732 m2. Three (3.48%), 2 (2.32%), 20 (23.25%), 18 (20.93%) and 43 (50%) had chronic kidney disease stage I, II, III, IV and V respectively.
Diabetic retinopathy
Of the 86 cases, 48 patients (55.81%) had DR and 38 patients (44.18%) had a normal fundus. Only 14 (37.5%) of 32 cases who had diabetes of <6 years duration, 17 (53.1%) of 32 cases who had diabetes of 6-10 years duration and 17 (77.2%) of the 22 cases who had diabetes of >10 years duration had DR. Prevalence of DR was more in patients who had diabetes for >10 years than patients who had diabetes for <6 years (P 0.022).
Histopathology
Out of 114 cases 86 (75.43%) cases had DN on histopathology. The most common histological class was Class IV observed in 37 (43.02. %) cases followed by Class III DN in 24 (27.90%) cases, Class IIa and Class IIb in 11 (12.79%) cases each and Class I DN in 3 (3.48%) cases.
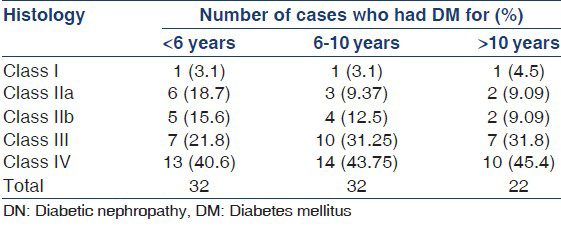
DN class versus duration of diabetes
Mean duration of diabetes in cases with DN was 7.31 ± 4.78 years. Mean duration of DM in DN Class I, IIa, IIb, III and IV was 6.5 ± 2.12, 6.25 ± 4.77, 5.36 ± 4.17, 7.72 ± 4.81 and 7.89 ± 5.15 years respectively. DN poorly correlated with duration of diabetes (P 0.336).
Of the three cases with Class I DN, one patient had DM of <6 years and one patient had DM for 8 years. 6 (54.5%) cases with Class IIa DN and 5 (44.45%) cases with Class IIb DN had DM for <6 years, only 2 (18.18%) cases with Class IIa and Class IIb had DM for >10 years. Of the cases with Class IV 13 (35.1%) cases had DM for <6 years, 14 (37.8%) cases had DM of 6-10 years and 10 (27.02%) cases had DM of >10 years duration. Duration of diabetes poorly correlated with Class of DN (P 0.85) [Table 1].
DN versus DR
Of the 86 cases 48 patients (55.81%) had DR and 38 patients (44.18%) did not have DR. DR poorly correlated with DN.
All three of Class I DN did not have DR, 27.2% of cases of Class II DN, 54 (58.33%) of Class III DN and 75.6% of cases with Class IV DN had DR respectively. Of the 48 cases with DR 58.33% of cases had Class IV DN, 14 (24.16%) cases had Class III DN, Class IIa and Class IIb was found in 3 (6.38%) cases each while none had Class I. Presence of DR correlated with higher class of DN (P = 0.012) [Table 2].

DN versus proteinuria
Mean 24 h urinary protein in patients with DN was 3.68 ± 2.44 g/day. Mean 24 h urinary protein in DN Class I, IIa, IIb, III and IV was 1.2 ± 1.27, 2.74 ± 3.33, 2.45 ± 1.26, 3.97 ± 1.87 and 4.31 ± 2.59 g/day respectively. Higher class of DN was associated with greater proteinuria (P 0.024).
DN versus hypertension
Of the 86 cases 48 (55.9%) cases had hypertension. 48.8% of cases with hypertension had Class IV DN on biopsy and 89.18% of cases with Class IV DN had HTN. Presence of hypertension correlated with higher class of DN (P 0.0003).
Correlation of eGFR and histology
Mean eGFR of cases in DN Class I, IIa, IIb, III and IV was 33.52 ± 24.9, 28.22 ± 21.9, 22.9 ± 8.6, 17.4 ± 19.6 and 10.12 ± 4.89 ml/min/1.732 m2 respectively. Mean eGFR correlated well with class of DN, with lower eGFR associated with higher DN class (P < 0.001).
Discussion
The progressive rise in the number of patients with ESRD due to DN is a major social and economic problem in several countries. Furthermore, prognosis in such patients is poor compared with patients with ESRD due to other renal diseases and hence special treatment guidelines are defined for this subset.[6] Proteinuria in diabetic patients is usually interpreted as a clinical manifestation of DN.[7] Although kidney biopsy is the most unbiased method of evaluation in proteinuric patients, it is rarely used in subjects with DM with isolated proteinuria.[8] The primary aim of kidney biopsy in proteinuric patients with DM is to confirm/exclude NDRD.
There are no standardized criteria for renal biopsy in DM or DN, therefore obtaining a renal biopsy from patients with DM or DN is currently a matter of judgment by the primary physician. Currently, renal biopsy is commonly performed in those patients who show features considered to be atypical for DN.[9] These include (1) absence of DR, (2) low or rapidly decreasing GFR, (3) rapidly increasing proteinuria or nephrotic syndrome, (4) presence of active urinary sediment or (5) signs or symptoms of other systemic disease. Biopsy is not indicated when there is typical evolution of renal disease and/or concomitant retinopathy.
Although pathologic classifications exist for several renal diseases, a uniform classification for DN is lacking. In 2010 Research Committee of the RPS developed a consensus classification combining type 1 and type 2 diabetic nephropathies.[5]
This study looked at the histology in patients with DN. In the literature we could not find many studies, which have classified DN according to this new classification. Ours is the first Indian study to classify cases of DN according to the RPS classification.
There are, however, multiple studies on NDRD. In the present study, NDRD on histopathology was present in 28 (26.9%) patients. The reported incidence of NDRD ranges from 23% to 54% in proteinuric type 2 DM patients.[1011121314151617] Variation in incidence could be due to selection bias in indications for biopsy. A meta-analysis of data available on prevalence of non-diabetic kidney disease among type 2 diabetic patients done by Zukowska-Szczechowska and Tomaszewski[18] revealed that non-DN was evident on kidney biopsy in approximately 22% of European and 26.7% of Asian patients with type 2 DM. Thus even after adjusting for differences in methodology among the studies, NDRD may affect a significant percentage of patients with type 2 DM. Therefore, kidney biopsy may become a useful diagnostic option among proteinuric patients of DM.
In the present study, mean duration of diabetes was 7.04 ± 4.9 years, mean duration of diabetes in cases with DN was 7.31 ± 4.78 years. The mean duration of DM in DN Class I, IIa, IIb, III and IV was 6.5 ± 2.12, 6.25 ± 4.77, 5.36 ± 4.17, 7.72 ± 4.81 and 7.89 ± 5.15 years respectively. There was no statistically significant correlation between duration of diabetes and class of DN (P = 0.83). However, Schwartz et al.[19] noted that there was significant difference in duration of diabetes between patients with Kimmelstiel-Wilson (K-W) lesions and mesangial lesions.
DN in cases of DR
In a study of renal biopsies in patients with presumed DN by Harada et al.[11] of the 21 cases with DR 18 (85.7%) had DN. Prakash et al.[10] reported 9 (60%) cases with retinopathy had DN and 40% had NDRD. In a study done by Christensen et al.[20] 20 of 52 patients with type 2 diabetes without DR and albuminuria more than 1 g/day were biopsied, out of which 35 (69%) patients had DN on biopsy. Schwartz et al.[19] noted 7 of 8 (87.5%) patients without retinopathy had mesangial sclerosis characteristic of DN. Prakash et al.[21] noted that 4 of 8 (50%) cases without DR had DN. Serra et al.[22] have reported that diabetic glomerulosclerosis was diagnosed in 17 (74%) of patients without DR. Thus, it is clear that absence of retinopathy cannot exclude the presence of DN. Clearly DN can occur in absence of retinopathy in type 2 proteinuric diabetic patients.
DR in patients of DN
In our study of the 86 cases of DN 48 patients (55.8%) had DR while 38 patients (44.18%) had a normal fundus. Presence or absence of DR poorly correlated with presence or absence of DN. In study by Harada et al.[11] of the 36 cases with biopsy proven DN 21 (58.33%) had DR while 15 (41.66%) did not have DR. Similarly 50% of proteinuric type 2 diabetic patients with typical DN on biopsy did not have DR in the study by Prakash et al.[21] Parving et al.[14] noted that DR was present in 15 of 27 patients (56%) with diabetic glomerulosclerosis, while none of the eight patients with a non-diabetic glomerulopathy had retinopathy.
In the present study, all three of Class I DN did not have DR while 27.2% of cases of Class II DN, 58.3% of Class III DN and 75.6% of cases with Class IV DN had DR respectively. Of the 48 cases with DR 58.3% of cases had Class IV DN, 14 (29.1%) cases had Class III DN, Class IIa and Class IIb was found in 3 (6.25%) cases each, none had Class I DN. Presence of DR correlated with higher class of DN (P 0.012). Similar observations were noted by Harada et al.[11] patients with both DN and DR showed more severe renal histology than those without DR. Schwatrz et al.[19] noted that patient with K-W lesions had correlation with retinopathy but not mesangial sclerosis, similar observations were made in type 1 DM also by Chavers et al.[23]
Mean 24 h urinary protein in NDRD was 2.3 ± 1.87 g/day. Higher class of DN was associated with higher proteinuria (P = 0.024). Similar observations were made by White[24] Osterby et al.[25] and Mise[26] where severity of proteinuria correlated with index of structural lesions.
Nearly 49% of cases with HTN had Class IV DN on biopsy and 89.3% of cases with Class IV DN had HTN. Presence of hypertension correlated with higher class of DN (P = 0.0003) in patients with DN. Mean eGFR in DN was 19.96 ± 17.52 ml/min/1.732 m2. Mean eGFR of cases in DN Class I, IIa, IIb, III and IV was 33.52 ± 24.9, 28.22 ± 21.9, 22.9 ± 8.6, 17.4 ± 19.6 and 10.12 ± 4.89 ml/min/1.732 m2 respectively. Lower eGFR was associated with higher DN Class (P < 0.001). Schwatrz et al.[19] noted that there was significant difference in creatinine clearance between patients with K-W lesions and mesangial lesions with lower GFR in the former group.
Thus, our study attempts to highlights the importance of renal histology in diagnosis and prognosis of diabetic kidney disease. A recent study has also shown that the prognosis of diabetic kidney disease depends on the histology and has validated the clinical utility of histological classification of DN.[27]
Conclusions
Most common histopathological lesion in patients with diabetes with renal dysfunction is DN. Among patients with DN most common class of DN is Class IV followed by Class III, II and Class I. Duration of diabetes correlated poorly with class of DN. However, eGFR and hypertension correlated well with histology with lower GFR and hypertension seen in higher histological classes. DR correlated poorly with presence or absence of DN. Thus, renal biopsy aids in staging of renal lesions in diabetic patients with renal dysfunction.
Acknowledgments
The authors wish to acknowledge Dr. Michelle and Dr. Meenakshi, Apollo Hospital, Hyderabad for analysis of renal histology, Dr. Superna Mahendra, Ophthalmologist, Sarojini Devi Eye Hospital, Hyderabad for eye examination and Dr. Balakrishna, N. Department of biostatistics National Institute of Nutrition for statistical analysis.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Diabetic Nephropathy. In: Maarten W. Taal, Glenn M. Chertow, Philip A. Marsden, Karl Skorecki, Alan S. L. Yu, Barry M, eds. Brenner and Rector's the Kidney (9th ed). Brenner Publisher Saunders; p. :1411-44. ISBN-13: 978-1416061939
- [Google Scholar]
- Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022-7.
- [Google Scholar]
- Diabetic nephropathy-Epidemiology in Asia and the current state of treatment. Indian J Nephrol. 2011;21:75-84.
- [Google Scholar]
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556-63.
- [Google Scholar]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-86.
- [Google Scholar]
- How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638-45.
- [Google Scholar]
- KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12-154.
- [Google Scholar]
- Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India. 2007;55:412-6.
- [Google Scholar]
- Significance of renal biopsy in patients with presumed diabetic nephropathy. J Diabetes Invest. 2013;4:88-93.
- [Google Scholar]
- Renal biopsy in patients with type 2 diabetes mellitus: Indications and nature of the lesions. Ann Saudi Med. 2009;29:450-3.
- [Google Scholar]
- Non-diabetic renal disease in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23:1000-7.
- [Google Scholar]
- Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758-62.
- [Google Scholar]
- Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458-66.
- [Google Scholar]
- A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23:1940-5.
- [Google Scholar]
- Nondiabetic kidney disease in type 2 diabetic patients: A single center experience. Indian J Nephrol. 2012;22:358-62.
- [Google Scholar]
- Renal affection in patients with diabetes mellitus is not always caused by diabetic nephropathy. Rocz Akad Med Bialymst. 2004;49:185-9.
- [Google Scholar]
- Renal pathology patterns in type II diabetes mellitus: Relationship with retinopathy. Nephrol Dial Transplant. 1998;13:2547-52.
- [Google Scholar]
- Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719-31.
- [Google Scholar]
- Non-diabetic renal disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2001;49:415-20.
- [Google Scholar]
- Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract. 2002;58:149-53.
- [Google Scholar]
- Relationship between retinal and glomerular lesions in IDDM patients. Diabetes. 1994;43:441-6.
- [Google Scholar]
- Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11:1667-73.
- [Google Scholar]
- Glomerular structure and function in diabetic nephropathy. Early to advanced stages. Diabetes. 1990;39:1057-63.
- [Google Scholar]
- Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143-55.
- [Google Scholar]
- Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant. 2014;29:109-18.
- [Google Scholar]







