Translate this page into:
Rituximab: A viable treatment option for epoetin-induced pure red cell aplasia
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pure red cell aplasia (PRCA) due to neutralizing antibodies can rarely develop following treatment with epoetin. The treatment of this condition is generally unsatisfactory and immunosuppression is often recommended, which improves chances of hematological recovery. We describe a case of PRCA due to neutralizing anti-epoetin antibodies following therapy with epoetin-α in a 68-year-old man on hemodialysis. He presented with severe transfusion-dependent anemia and was initially treated with prednisolone and oral cyclophosphamide. However, within 2 weeks the immunosuppressive drugs had to be stopped due to complications, following which he remained transfusion dependent. Subsequently, he was given two doses 700 mg each of rituximab following which there were hematological recovery and resolution of anti-epoetin antibodies.
Keywords
Anti-epoetin antibodies
pure red cell aplasia
rituximab
Introduction
Pure red cell aplasia (PRCA) is a rare disorder characterized by maturation arrest of erythrocytes, normocytic normochromic anemia, severe reticulocytopenia, almost complete absence of marrow erythroblasts, and normal production of white cell and platelets.[1] PRCA causes severe, transfusion-dependent anemia. PRCA in adults is generally acquired, can be primary or secondary and often the cause is immunological. The secondary causes of PRCA are drugs, viral infections, hematological malignancies, systemic lupus erythematosis and solid tumors. It is rarely seen as an adverse effect of long-term use of recombinant human erythropoietin (epoetin) due to the production of neutralizing anti epoetin antibodies.[2] Anti-epoetin antibody-mediated PRCA is suspected when epoetin is used for some length of time, manifests as rapid onset of severe anemia, which requires transfusions to maintain stable hemoglobin (Hb). The definitive diagnosis, however, requires two criteria: (1) bone marrow examination showing normal cellularity and almost absence of erythroblasts, and (2) demonstration of neutralizing antibodies against epoetin in the blood. In addition, serum erythropoietin levels tend to be low in anti-epoetin antibody-mediated PRCA in contrast to higher levels seen in other conditions causing PRCA such as hematological malignancies. In 1997, following a decade of widespread clinical use after introduction in 1986, definitive cases of epoetin-associated PRCA was reported.[2] Subsequently, the reported cases increased progressively to peak in 2002 and then showed a decline since 2003. The cause of increased incidence of epoetin-associated PRCA during this period remains unclear. However, several factors such as formulations lacking in human serum albumin, subcutaneous administration and uncoated rubber stoppers used in prefilled syringes were thought to have contributed to this epidemic.[2]
The optimal treatment of anti-epoetin antibody-mediated PRCA is unclear. Mere withdrawal of epoetin is mostly unsatisfactory and immunosuppressive therapy appears to enhance markedly the likelihood of recovery.[3] Several immunosuppressive drugs such as corticosteroids, cyclophosphamide, cyclosporine and intravenous immunoglobulin (IV Ig) have been used with variable reported response.[4] Renal transplantation results in resolution of the condition in almost all cases.[3] Very few case reports of PRCA treated with rituximab appear in the literature.
We report a case of anti-epoetin antibody-mediated PRCA, treated successfully with rituximab, following intolerance to corticosteroid and oral cyclophosphamide.
Case Report
A 68-year-old man was diagnosed to have end-stage renal disease due to hypertensive nephropathy and was initiated on hemodialysis (HD) in March 2012. He received HD three times a week for 5 h each time. He received epoetin-α 4000 IU twice a week intravenously initially and later switched to subcutaneous administration in August 2013. He responded well to epoetin till November 2013, when Hb was 11.6 g/dl. Between November 2013 and January 2014, he had a precipitous drop in Hb (11.6 to 6.4 g/dl) for no obvious reason. Iron deficiency, external bleeding, gastrointestinal blood loss or hemolysis was excluded by appropriate investigations. Anemia worsened rapidly over next few days and he was hospitalized with significant anemic symptoms and received 4 units of blood during the hospital stay. Erythropoietin treatment was withdrawn as epoetin related PRCA was suspected since reticulocyte count was low at 0.7%. Bone marrow aspiration and biopsy were done, which confirmed the presence of PRCA. Bone marrow showed normocellular marrow, with severe suppression of erythroid series and normal myeloid and megakaryocyte series [Figure 1]. The presence of neutralizing anti-epoetin antibodies was confirmed by immunoprecipitation test [Table 1]. The neutralizing anti-epoetin antibody was positive at a dilution of 1:10,000 at the time of diagnosis. Serum level of endogenous erythropoietin levels was low [Table 1]. Renal transplantation was not a viable option for him in view of advanced age and comorbidities. In an effort to reduce the anti-epoetin antibodies, he was commenced on oral cyclophosphamide (1.5 mg/kg/day) and oral prednisolone 0.5 mg/kg/day. Two weeks into the treatment, he developed massive gastrointestinal bleed due to duodenal ulcer, requiring blood transfusions, following which steroid was withdrawn. He was admitted to Critical Care Unit where he developed right upper zone pneumonia requiring mechanical ventilation. In addition, he developed low white cell count, necessitating withdrawal of cyclophosphamide. He was given 8 units of transfusions during 10 days of hospitalization. Subsequently he remained transfusion dependent and required approximately one unit of red cells per week to maintain Hb above 6 g/dl [Figure 2]. In view of advanced age and intolerance to conventional immunosuppressive agents such as steroid and cyclophosphamide, he was treated with rituximab, 2 doses of 700 mg (approximately 500 mg/m2) in the month of April 2014, given 2 weeks apart. Meanwhile, he was also treated with 3 doses of androgenic steroid (nandrolone 25 mg) at 15 days interval, in order to support the recovery of the bone marrow. With the treatment, his Hb remained in the range of 6.1–6.4 g/dl for 2–3 weeks without blood transfusion and subsequently improved to above 7 g/dl. Currently, the patient maintains his Hb above 7 g/dl without any need for blood transfusion for more than 6 months after the last dose of rituximab. Serial measurement of neutralizing anti-epoetin antibody titer showed a decline and reached nonsignificant levels by 6 months, demonstrating success with therapy [Table 1]. We did not repeat the bone marrow study since there was clear clinical evidence of hematological recovery in the form of sustained increment in Hb, freedom from a blood transfusion and an increase in the reticulocyte count. He was not rechallenged with any other preparation of epoetin since he was symptom-free and did not require any further blood transfusions to maintain stable Hb.
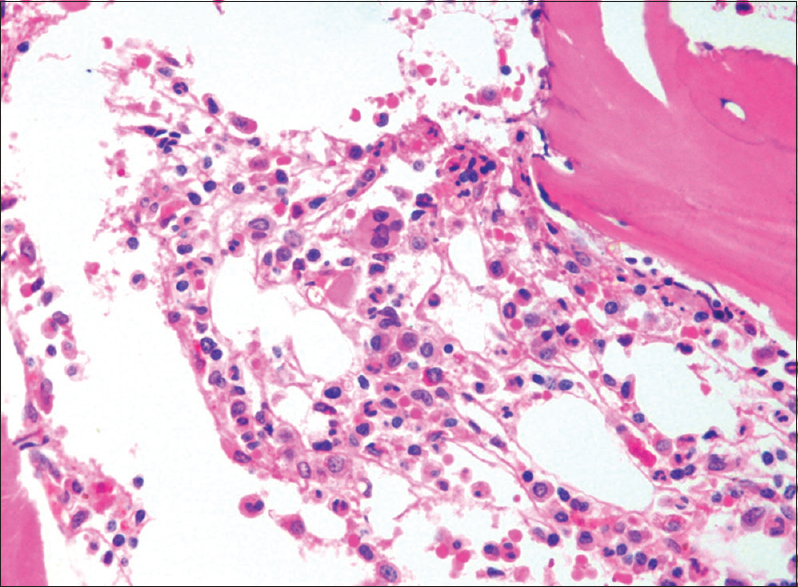
- Bone marrow biopsy showing absence of erythroblasts and normal myeloid series and platelet precursors


- Hemoglobin and number of red cell transfusions over time and their relation to treatment. White arrow depicts duration of treatment with corticosteroid and oral cyclophosphamide. Black arrow represents two doses of rituximab
Discussion
We present a case of PRCA who responded to rituximab, indicating successful treatment of the condition. He also received three doses of nandrolone 25 mg each, which, however, is unlikely to have caused a sustained increment in Hb. The use of anabolic steroid may have some benefit in elderly males with anemia who are likely to have testosterone deficiency, but its benefit in renal anemia is doubtful and is not recommended for use in chronic kidney disease (CKD).[5]
Treatment of anti-epoetin antibody-mediated PRCA is unsatisfactory despite the withdrawal of the drug. Verhelst et al. reported that hematological recovery was seen only in 2% of the patients after withdrawal of the drug, whereas immunosuppressive therapy improved the likelihood of recovery to 52%.[3] Since no randomized trials are available to compare individual immunosuppressive drugs, the data is insufficient to provide clear guidelines for a preferred agent to treat this condition. However, amongst the immunosuppressive agents, the hematological recovery is the highest in those treated with steroid and cyclophosphamide (87%) and lowest with IV Ig (11%). Monotherapy with cyclosporine or steroid has intermediate efficacy.[3]
Rituximab is a genetically engineered monoclonal antibody directed against CD20 antigen, seen in certain subset of B-cells and causes significant B-cell depletion.[6] It is being increasingly used to treat several immune mediated diseases. It is an expensive drug and it remains to be understood how best to use it in cost-effective manner. Case reports and case series have reported successful treatment of PRCA with rituximab in non-epoetin related PRCA such as lymphoproliferative malignancies[7] and primary PRCA.[8] Rituximab offers an attractive alternate to other immunosuppressive agents to treat epoetin related PRCA. However, rituximab is not commonly used and only few cases of rituximab used to treat epoetin related PRCA are reported in the literature.[391011] Behler et al. reported a case of epoetin related PRCA in a woman receiving ribavirin and interferon for hepatitis C infection.[9] She received a high dose of epoetin alfa 40,000 units per week for treatment of anemia and developed neutralizing antibodies against epoetin. She was treated with rituximab and IV Ig, which resulted in hematological recovery and disappearance of neutralizing anti-epoetin antibodies. Treatment with rituximab did not cause reactivation of hepatitis C infection. Mandreoli et al. reported success with rituximab to treat PRCA due to epoetin alfa in an 80-year-old patient with CKD.[10] Conventional immunosuppressive therapy was deferred for practical reasons in this patient, due to advanced CKD and age. Comont et al. reported a case of 69-year-old male with CKD and myelodysplastic syndrome on epoetin-α who developed epoetin induced PRCA. Steroids, cyclophosphamide, and cyclosporine were considered unsuitable due to comorbid conditions and hence he was given 2 g of IV rituximab, following which anti-epoetin antibodies reduced substantially after a month.[11] In contrast, Verhelst et al. reported no response to rituximab in 2 patients treated with rituximab in the case series of epoetin related PRCA.[3] In summary, of the 5 cases of epoetin related PRCA treated with rituximab previously reported in the literature, 3 showed success while, other 2 showed no benefit. Our case and few other reports suggest that rituximab is a viable alternative in treating PRCA due to the neutralizing anti-epoetin antibody. Extensive clinical experience in several hematological malignancies and immune-mediated diseases suggest that rituximab is safe and generally well tolerated even in patients with renal dysfunction.[6] It may be used an alternative to currently established immunosuppressive therapy such as steroid and cyclophosphamide if these drugs are contraindicated or not tolerated. However, the high cost of rituximab may be a deterrent to use it as afirst-line therapy, especially in developing world.
Conclusion
We report a case of anti-epoetin antibody-mediated PRCA in a 68-year-old man, which was successfully treated with rituximab resulting in hematological recovery and complete disappearance of neutralizing anti-epoetin antibodies. Our case suggests that rituximab offers a viable alternative to treat PRCA due to epoetin, in whom alternate immunosuppressive agents are not tolerated or contraindicated.
Acknowledgment
We are grateful to Dr. David Wilkinson, CLIA Immunochemistry Laboratory, Richmond, VA, USA and Janssen, a Pharmaceutical division of Johnson and Johnson Private Limited for the serial measurements of neutralizing anti-epoetin antibody.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Pure Red Cell Aplasia Global Scientific Advisory Board (GSAB). Erythropoietin-induced, antibody-mediated pure red cell aplasia. Eur J Clin Invest. 2005;35(Suppl 3):95-9.
- [Google Scholar]
- Epoetin-associated pure red cell aplasia in patients with chronic kidney disease: Solving the mystery. Nephrol Dial Transplant. 2005;20(Suppl 3):iii33-40.
- [Google Scholar]
- Treatment of erythropoietin-induced pure red cell aplasia: A retrospective study. Lancet. 2004;363:1768-71.
- [Google Scholar]
- Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: A follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project. In: Blood. Vol 106. 2005. p. :3343-7.
- [Google Scholar]
- KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013;62:849-59.
- [Google Scholar]
- Update on rituximab: An established treatment for all immune-mediated kidney diseases? Nephron Clin Pract. 2014;126:97-109.
- [Google Scholar]
- Pure red cell aplasia in B-cell lymphoproliferative disorder treated with rituximab: Report of two cases and review of the literature. Leuk Res. 2006;30:109-14.
- [Google Scholar]
- Restoration of erythropoiesis by rituximab in an adult patient with primary acquired pure red cell aplasia refractory to conventional treatment. Br J Haematol. 2002;116:727-8.
- [Google Scholar]
- Rituximab therapy for pure red cell aplasia due to anti-epoetin antibodies in a woman treated with epoetin-alfa: A case report. J Med Case Rep. 2009;3:7335.
- [Google Scholar]
- Successful resumption of epoetin alfa after rituximab treatment in a patient with pure red cell aplasia. Am J Kidney Dis. 2004;44:757-61.
- [Google Scholar]
- Rituximab in pure red-cell aplasia secondary to anti-erythropoietin antibodies. Kidney Int. 2014;86:210-1.
- [Google Scholar]







