Translate this page into:
Splenorenal graft: A safe and effective treatment for renovascular hypertension
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renovascular hypertension can be managed medically in most cases. However, in cases of failed medical therapy revascularization is indicated. Splenorenal grafting is one such method for revascularization. We present a report of splenorenal grafting for the management of resistant hypertension.
Keywords
Renal artery stenosis
renal vascularization
renovascular hypertension
splenorenal bypass
Introduction
Renovascular hypertension (RVH) due to renal artery stenosis (RAS) is an important cause of severe hypertension. Fibromuscular dysplasia (FMD), Takayasu's arteritis, middle aortic syndrome and atherosclerosis are the common causes of RAS. Treatment is directed toward the control of hypertension and preservation of renal function. Revascularization either by percutaneous angioplasty with or without stenting or by open surgical methods may be needed if medical therapy fails to control hypertension or there is progressive deterioration of renal function. Among revascularization procedures, splenorenal anastomosis is safe and effective for management.
Case Report
A 17-year-old male presented with shortness of breath, swelling of feet and oliguria of 2 months duration. He was found to have severe hypertension with impairment of renal functions and pulmonary edema. Pulse rate was 80 min, regular. Blood pressure (BP) in right upper and lower limbs were 270/120 mmHg and 210/120 mmHg, and the left upper and lower limb was 260/140 mmHg and 260/130 mmHg respectively. Abdominal examination revealed bilateral systole-diastolic renal bruit. Fundus examination showed hypertensive neuroretinopathy. Investigations showed serum creatinine 2.1 mg/dl, blood urea 62 mg/dl, sodium 139 mmol/l, potassium 3.1 mmol/l, blood sugar 86 mg/dl, haemoglobin 9.4 g/dl, total leucocyte count 7200/mm3, erythrocyte sedimentation rate: 20 mm, serum uric acid 6.6 mg/dl, serum calcium 10.6 mg/dl, serum phosphorus 4.9 mg/dl, serum proteins: 7.1 g/dl, serum albumin 3.1 g/dl. Thyroid function tests were normal. Urine examination showed protein: +, 4–6 white blood cell/hpf and 2–4 red blood cells/hpf. Twenty-four hours urine examination showed a total urine volume of 4900 ml. Urine protein: creatinine ratio was 0.27.
Ultrasound showed right kidney measuring 8.9 cm × 3.4 cm and left kidney 9.2 cm × 4.0 cm with normal echotexture.
Two-dimensional echocardiogram revealed moderate concentric left ventricular hypertrophy. Left atrium was dilated, valves were normal. There was aortic regurgitation, moderate left ventricular systolic dysfunction and Grade I left ventricular diastolic dysfunction.
Renal Doppler showed diffuse wall thickening with more than 90% diffuse luminal stenosis bilaterally. Distal renal arteries were poorly visualized and show decreased flow velocities. Intrarenal parenchymal arteries showed reverse flow pattern. Right renal artery showed a peak systolic velocity of 31 cm/s while the left renal artery had a peak systolic velocity of 46 cm/s. Proximal aorta showed diffuse wall thickening and decreased caliber (aorto arteritis with B/L proximal RAS of > 90%).
Computed tomography (CT) angiogram showed 60–70% stenosis of renal arteries at origin bilaterally [Figure 1]. Proximal abdominal aorta showed decreased caliber with thickened wall. The coronary arteries were normal.
- Bilateral renal artery stenoses
Conventional angiogram showed diffuse concentric thickening of distal thoracic and upper abdominal aorta with bilateral long-segment occlusion of main renal arteries with distal renal arteries filling through collaterals.
Patient was on clonidine 0.2 mg tid, nifedipine retard 20 mg tid, prazosin XL 5 mg bid, carvedilol 3.125 mg bid and torsemide 20 mg bid. With above medications, BP was 160/110 mmHg. The patient was initially treated with steroids, but there was no improvement.
As both renal arteries had long-segment stenosis proximally with good distal caliber, splenrenal arterial anastomosis was planned. Intraoperative findings revealed that left renal artery was replaced by fibrous cord till hilum. One centimeter stump of the renal artery (6 mm diameter) was felt in the hilum, dividing into upper and lower divisions. The abdominal aorta was encased by thick inflammatory tissue and lymph nodes. The splenic artery was identified and anastomosed with left renal artery end to end (splenorenal anastomosis).
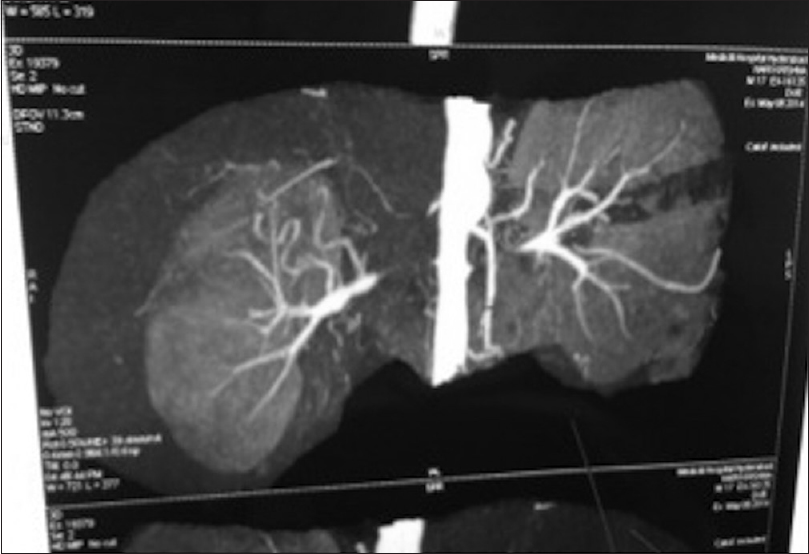
On 2nd postoperative day BP was 140/84 mmHg with nifedipine 20 mg 1 tablet tid. Postoperative Doppler showed good filling of left distal renal artery through the splenic artery with good renal perfusion. CT angiogram was performed on 10th postoperative day that demonstrated patent splenorenal arterial anastomosis [Figure 2]. Patient was discharged on 10th postoperative day with advice for right kidney revascularization after 2 months. Serum creatinine at discharge was 1.2 mg/dl. At 6 months, postoperation patient's BP was 120/80 without any drugs.

- Splenorenal anastomosis
Discussion
The common causes of RVH in our country are Takayasu's arteritis, FMD and atherosclerosis.[1] RVH is amenable to treatment with anti-hypertensive drugs, and revascularization is required only for uncontrolled hypertension or for the preservation of renal function. There are numerous techniques for renal artery revascularization. Percutaneous angioplasty with or without stenting has been described.[2] Percutaneous transluminal renal angioplasty (PTA) has good success especially for FMD but in extensive aortic disease or bilateral stenosis PTRA is not used. Open surgical techniques include aortic bypass e.g., aortorenal bypass using saphenous vein, hypogastric artery or a prosthetic graft. In patients with difficult aortic surgery, splenorenal arterial anastomosis, hepatorenal or mesenteric-renal bypass can be done. Other revascularization techniques include endarterectomy and autotransplant. The arterial wall may be friable in the acute stage hence surgery should be avoided in the acute stage.
Selection of technique depends on each individual case, the clinical situation, and the preference of the individual surgeon. In addition, the status of the contralateral renal artery and the existence of any other vascular lesion must be evaluated, particularly associated arteriosclerotic disease of the abdominal aorta.[3]
Aortorenal bypass involves greater risk of dissection and the use of a bypass graft.[34] Autogenous saphenous vein, hypogastric artery or a prosthetic graft may be employed, depending on individual requirements and preference of the surgeon. Use of polytetrafluoroethylene as graft material increases the risk of thrombosis and infection; use of saphenous vein is technically difficult because of its small size (2 mm diameter) and has been associated with aneurysmal degeneration and sub-intimal hyperplasia. Occasionally, saphenous vein may be absent or inadequate in caliber, or in some instances may be better preserved for possible future use in aortocoronary or femoropopliteal reconstructions. Autogenous hypogastric artery represents an ideal graft material, but its usefulness is often limited by associated arteriosclerosis and the requirement for dissection in a far removed operative field.
Splenic artery bypass provides an autologous arterial connection. This procedure was reported in 1950s.[5] Since that time scattered reports have appeared, the largest of which is survey by Khauli et al.[6] Kaufman[7] and Myers and Johnson found it technically challenging;[8] however, reports by Brewster et al. show that it is a simple and safe procedure. The risk of infection and thrombosis is reduced by avoiding the use of foreign materials/artificial grafts. Use of an arterial conduit decreases the potential for aneurysmal degeneration. The splenic artery is of comparable size to renal artery. The technique is efficient, requiring only one vascular anastomosis. Difficulties with a diseased aorta are avoided. The procedure is suitable for a staged approach to bilateral disease or in reoperation for failed prior reconstructions. In a paper by Brewster et al., the procedure was reviewed in 19 patients. There were no deaths, and a single failure occurred due to graft occlusion (5%). Of the remaining 18 patients, 7 were cured and 11 improved. There were no cases of postoperative renal failure, and renal function improved in all four patients with renal failure preoperatively. In selected patients, this procedure is of great usefulness and deserves wider application.[91011] Good preoperative angiographic evaluation of coeliac axis and splenic artery is needed when splenorenal graft is considered. Adequate lateral and often oblique films should be obtained to exclude any associated lesions that may contraindicate the use of splenic artery. Splenectomy is not needed as spleen is adequately supplied by the short gastric vessels.[91011] Brewster et al. recommend an end-end technique while Novick et al. favored the end-side method.[12] Multiple studies have demonstrated the efficacy of spenorenal grafting in cases of uncontrolled hypertension. The technique is effective, safe and requires a single vascular anastomosis. In our case, this technique was chosen as the diseased aorta and long-segment proximal stenosis of renal arteries precluded the use of these vessels.
Endarterectomy may be employed for focal arteriosclerotic lesions; however, it is not suited to stenoses associated with significant aortic arteriosclerosis and is inapplicable in FMD.[3] Nephrectomy is the last resort for a nonsalvageable kidney or a nonreconstructable situation.
Conclusion
In this case report, we highlight the role of splenorenal bypass in the management of refractory RVH.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Nonspecific aortoarteritis. In: Rutherford RB, ed. Vascular Surgery. Philadelphia: WB Saunders; 1989. p. :217-37.
- [Google Scholar]
- Survival and quality of life after renal angioplasty: A five-year follow-up study. Scand J Urol Nephrol. 2009;43:236-41.
- [Google Scholar]
- Splenorenal arterial anastomosis for renovascular hypertension. Ann Surg. 1979;189:353-8.
- [Google Scholar]
- Aortitis syndrome due to Takayasu's disease. A guideline for the surgical indication. J Cardiovasc Surg (Torino). 1976;17:443-56.
- [Google Scholar]
- Severe hypertension due to congenital stenosis of artery to solitary kidney; correction by splenorenal arterial anastomosis. AMA Arch Surg. 1957;75:1023-6.
- [Google Scholar]
- Splenorenal bypass in the treatment of renal artery stenosis: Experience with sixty-nine cases. J Vasc Surg. 1985;2:547-51.
- [Google Scholar]
- Dacron grafts and splenorenal bypass in the surgical treatment of stenosing lesions of the renal artery. Urol Clin North Am. 1975;2:365-80.
- [Google Scholar]
- The surgical treatment of renovascular hypertension 1.Selection and techniques. Med J Aust. 1971;1:1305-11.
- [Google Scholar]
- Surgical treatment of renal hypertension: Results in patients with occlusive lesions of renal arteries. J Urol. 1959;82:403-11.
- [Google Scholar]
- Revascularization of the kidney in hypertension due to renal artery stenosis. AMA Arch Surg. 1959;79:269-75.
- [Google Scholar]
- Splenorenal bypass in the treatment of stenosis of the renal artery. Surg Gynecol Obstet. 1977;144:891-8.
- [Google Scholar]







