Translate this page into:
Lupus podocytopathy: An important differential diagnosis of nephrotic syndrome in systemic lupus erythematosus
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Some patients with systemic lupus erythematosus (SLE) present with sudden onset of nephrotic syndrome and biopsy findings may be of minimal change disease or focal segmental glomerulosclerosis with diffuse foot process effacement on electron microscopy but without significant immune deposits. This entity is termed lupus podocytopathy. Clinicians and renal pathologists need to be aware of this condition. Though steroid sensitive, it needs follow-up to recognize flare and class change, thereby optimizing therapy.
Keywords
Electron microscopy
lupus podocytopathy
systemic lupus erythematosus
Introduction
The classification of lupus nephritis (LN) was revised by the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) in 2003. Immune complex aggregation is central to LN and universally present in all subclasses. The increasingly recognized phenomenon of apparent minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) without significant immune deposits in a patient with systemic lupus erythematosus (SLE) is termed as lupus podocytopathy (LP).[1] The diagnosis of LP in a patient with SLE and nephrotic syndrome is based on the finding of severe foot process effacement in the absence of peripheral capillary wall immune deposits.[2] LP has not been included in the current ISN/RPS classification. We report two cases of LP in patients with SLE.
Case Report
A 22-year-old female presented with fever, symmetrical arthritis involving small and medium joints of 3 months duration. She had oral ulcers, malar rash, and pedal edema Urinanalysis revealed 3+ protein, 2–4 red blood cells/hpf, 1–2 white blood cells (WBCs)/hpf, no casts or crystals. Her 24 h urine protein was 3.8 g/day. Hemogram showed hemoglobin 9.2 g/dl, total WBC count 4800/µl, and platelets 2.2 lakhs/µl. Serum creatinine was 0.9 mg/dl. Serum protein and albumin were 6 g/dl and 3.0 g/dl, respectively. Total cholesterol was 301 mg/dl and triglycerides 250 mg/dl. Serology was positive for anti-nuclear antibodies (ANA) and antidsDNA. Lupus anticoagulant, and anticardiolipin antibodies were absent. Serum C3 was 60 mg/dl (normal 90–180 mg/dl), C4-10 mg/dl (normal 10–40 mg/dl). Prothrombin time/international normalized ratio, activated partial thromboplastin time were normal. She satisfied five out of 11 ACR criteria for SLE. She denied renal biopsy. She discontinued her medications after 2 weeks. She was admitted after 3 months for persistent edema and underwent renal biopsy that showed morphologically normal glomeruli with normal appearing capillary loops, mesangium, tubulointerstitium, and blood vessels. Immunofluorescence study was negative for IgG, IgM, IgA, C3, C1q, Kappa, and lambda light chains. Electron microscopy (EM) showed diffuse foot process effacement with no electron dense deposits in mesangial, capillary walls, or tubulointerstitial compartment [Figure 1]. These features were consistent with MCD. She was started on oral prednisolone 50 mg/day, and attained complete remission in 4 weeks. Prednisolone was tapered after 8 weeks. After 27 months of follow-up, she maintains remission, off prednisolone.
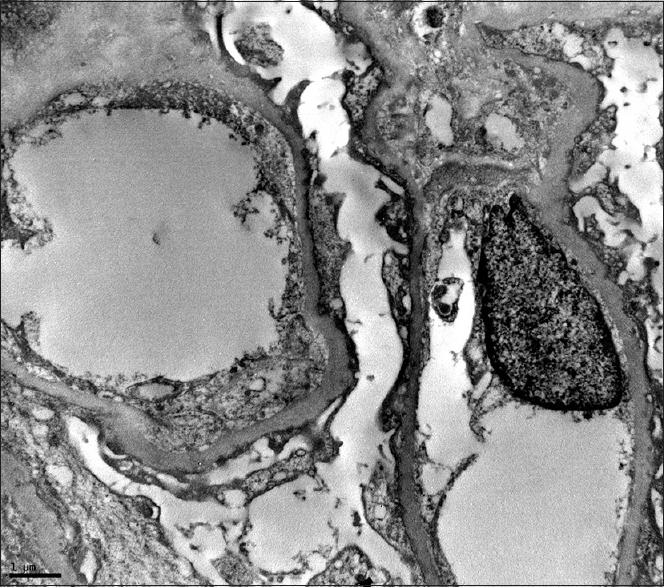
- Electron micrograph of a portion of a glomerulus shows diffuse effacement of foot processes of podocytes. Note that there are no electron dense deposits in the subepithelial, subendothelial or mesangial areas
The second case is a 36-year-old male who presented with anasarca and arthritis involving small and large joints. There was no history of fever, rash, oral ulcer, photosensitivity, or hair loss. Clinical examination revealed mild anemia, anasarca, hypertension, and evidence of active small joint arthritis. Urine showed 3+ protein, with 11.5 g/day proteinuria. His creatinine was 2.63 mg/dl with serum albumin of 2.2 g/dl and cholesterol 330 mg/dl, and triglycerides of 380 mg/dl. Hemogram showed normochromic normocytic anemia with normal total leukocyte count and platelet count. Serology was positive for ANA and anti-double-stranded DNA with low C3 (46 mg/dl) and borderline low C4 (8.43 mg/dl). Serology was negative for rheumatoid factor, antibodies to cyclic citrullinated peptide, hepatitis B virus, hepatitis C virus and HIV as well as anti-proteinase 3 and anti-myeloperoxidase. Nephrotic range proteinuria, anemia, arthritis, ANA positive serositis and serology profile satisfied SLICC diagnostic criteria and patient was subjected to renal biopsy. Histopathological examination showed a total of 14 glomeruli. Segmental sclerosis with adhesions to the Bowmans capsule was seen in two glomeruli [Figure 2]. The tubulointerstitial and vascular compartment were unremarkable. Immunofluorescence study was negative for IgG, IgM, IgA, C3, C1q, kappa, and lambda light chains. There was diffuse foot process effacement with no electron dense deposits in the mesangial region, capillary walls or tubular compartment on EM. The above features were consistent with FSGS, not otherwise specified, in a patient with SLE. He was treated with pulse methylprednisolone for 3 days and IV diuretic with albumin, followed by oral steroids after 3 days 1 mg/kg body weight, with rapid resolution of edema and normalization of serum creatinine. At 2 weeks, proteinuria came down to 4 g/day and by 12 weeks he was in partial remission with <2 g proteinuria. After 12 months follow-up, he remains in complete remission, but on 5 mg of prednisolone per day.
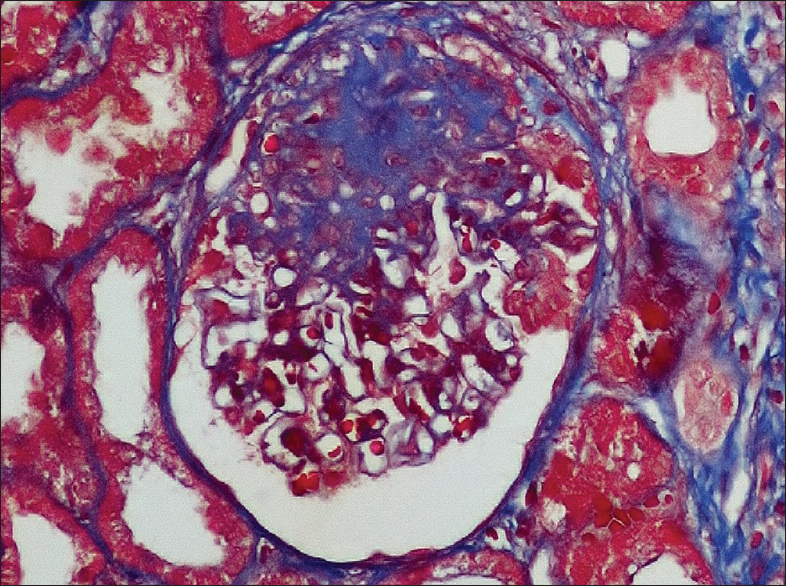
- Segmental sclerosis with adhesions to the Bowman's capsule (Trichrome, ×400)
Discussion
These two case reports describe nephrotic syndrome in patients with active SLE. Immune complex aggregation that is thought to be a key feature of SLE was conspicuously absent in our SLE patients. The light microscopic findings with absence of immune deposits on immunofluorescence study and diffuse foot process effacement on EM with rapid remission of nephrotic syndrome with prednisolone support the diagnosis of MCD and FSGS associated with active extra renal lupus. It is important to follow-up these cases whether this proteinuria though steroid responsive, has a potential to flare or change class to typical LN in later days.
The first question remains whether this entity is a chance occurrence in SLE and if it is not, to explain this by pathogenic mechanism common to SLE.
The incidence of nephrotic syndrome in LN is 40% to 65%.[1] The presence of minimal or no capillary wall immune deposits with or without mesangial proliferation associated with nephrotic proteinuria is collectively termed as LP.[2] In literature, so far 22 cases have been reported.[34567891011121314] Most literature quote that LP is associated with Class II LN. The association of MCD or FSGS without immune deposits in SLE is of a rarity. The chance occurrence of MCD and SLE in the same individual is expected to be <1 in 10,000.[13] Dube et al.[3] in a series of seven patients with LP, reported two patients with features consistent with MCD akin to our case. Horino et al.[14] reported the case of a 29-year-old SLE patient with remitting and relapsing nephrotic syndrome. Her renal biopsy showed normal glomeruli with effacement of foot processes compatible with MCD, which completely remitted without further relapse. Hertig et al.[13] reported 11 SLE patients with idiopathic nephrotic syndrome on biopsy with active SLE. Kraft et al.[4] evaluated a series of 18 renal biopsies from a series of 470 patients, eight with nephrotic range proteinuria and 10 from nonnephrotic proteinuria. Seven of eight biopsies with nephrotic range proteinuria showed more than 80% foot process effacement while no biopsy for nonnephrotic range proteinuria had exhibited more than 20% foot process effacement. In this study, only eight biopsies fit into LP associated with nephrotic syndrome.
To explain the pathogenesis, the majority of authors[1516171819] favor a circulating toxin hypothesis, though the exact agent has never been proved. Interleukin (IL)-13 has been suggested on the basis of overexpression in rats causing apparently inducing minimal change picture on EM.[18] The IL-13 pathway has also been implicated to have a role in Hodgkin's lymphoma where MCD has been found in high prevalence. Aberrant T cell function is responsible for the podocyte injury in MCD.[1516] Abnormal release of IL-13 from aberrant T-cells and a cross talk between the renal dendritic cells and the Th cells may directly damage the podocytes.[17] Animal studies with rats showed IL-13 overexpression leading to podocyte injury with downregulation of nephrin, podocin and dystroglycan and concurrent upregulation of B7-1 in the glomeruli inducing MCD.[18] T cell function plays an important role in the pathogenesis of SLE by altering the expression of adhesion molecules as well as inducing B cells to produce autoantibodies.[4] Ineffective immune regulation in SLE is due to a functional imbalance of T lymphocyte subsets. Activated Th2 subset overproduces IL-6, IL-10, IL-13, and tumor necrosis factor-α whereas Th1 subset under produces IL-2, interferon-γ and transforming growth factor-β. IL-10 and IL-13 play an important role in Th2 cell differentiation and production of autoantibodies in SLE. The T-cell abnormalities in both the disorders could be the unifying pathogenic mechanism in the occurrence of MCD or FSGS in SLE.
In our patients, proteinuria responded to steroid therapy but one of them is still on minimal steroid dose. These patients need longer follow-up, with the need for repeat biopsy with a nephrotic or nephritic flare if any.
Conclusion
LP should be recognized as a part of the renal disease spectrum of SLE. Diffuse podocyte effacement without immune complex deposition on EM is the sine qua non for this entity. Clinicians and renal pathologists need to be aware of this entity as a cause of nephrotic syndrome in patients with SLE. Importantly, this lesion should be recognized as highly steroid responsive but a vigilant follow-up is needed keeping in mind the natural history of flare and quiescence of this disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal involvement in systemic lupud erythematosus (SLE): a study of 56 patients emphasizing histologic classification. Medicine (Baltimore). 1978;57:371-410.
- [Google Scholar]
- Heptinstalls Pathology of the Kidney (7th ed). Philadelphia, USA: Wolters Kluwer; 2015. p. :608-9.
- Minimal change disease in systemic lupus erythematosus. Clin Nephrol. 2002;57:120-6.
- [Google Scholar]
- Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2005;16:175-9.
- [Google Scholar]
- Three cases presenting with systemic lupus erythematosus and minimal change nephrotic syndrome. Nihon Jinzo Gakkai Shi. 1989;31:991-9.
- [Google Scholar]
- A case report of lupus nephritis associated with minimal change nephrotic syndrome – Comparison of various histological types of 67 cases with lupus nephritis. Nihon Jinzo Gakkai Shi. 1992;34:835-40.
- [Google Scholar]
- Minimal-change nephrotic syndrome associated with systemic lupus erythematosus. Am J Nephrol. 1995;15:439-41.
- [Google Scholar]
- Systemic lupus erythematosus in a patient with remitting minimal change nephrotic syndrome. Clin Nephrol. 1997;48:327-30.
- [Google Scholar]
- Minimal-change nephropathy associated with systemic lupus erythematosus. Nephrol Dial Transplant. 1997;12:2030-1.
- [Google Scholar]
- A case of systemic lupus erythematosus associated with minimal change nephrotic syndrome. Nihon Jinzo Gakkai Shi. 1997;39:759-64.
- [Google Scholar]
- A case of minimal change nephrotic syndrome manifesting acute renal failure in the course of systemic lupus erythematosus. Nihon Jinzo Gakkai Shi. 2002;44:476-82.
- [Google Scholar]
- Acute renal failure associated with a minimal change nephrotic syndrome in a systemic lupus erythematosus patient. Yonsei Med J. 2002;43:114-8.
- [Google Scholar]
- SLE and idiopathic nephrotic syndrome: coincidence or not? Am J Kidney Dis. 2002;40:1179-84.
- [Google Scholar]
- Minimal change nephrotic syndrome associated with systemic lupus erythematosus. Nephrol Dial Transplant. 2006;21:230.
- [Google Scholar]
- Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet. 1975;2:556-60.
- [Google Scholar]
- Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286-97.
- [Google Scholar]
- Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476-85.
- [Google Scholar]
- Hypomethylation of IL10 and IL13 promoters in CD4+ T cells of patients with systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:931018.
- [Google Scholar]







