Translate this page into:
Hyponatremia - A rare complication of Gitelman's syndrome
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Gitelman's syndrome (GS) is a rare autosomal recessive disorder caused by mutations in thiazide-sensitive NaCl cotransporter. We report a 49-year-old, normotensive lady with prolonged hypokalemia since her 20s who was diagnosed with GS at our renal clinic. During follow-up, she was found to have mild, asymptomatic, euvolemic hyponatremia with low serum uric acid, inappropriately high urine osmolality and sodium consistent with syndrome of inappropriate antidiuretic hormone-like presentation. Despite life-long urinary sodium losses, hyponatremia has rarely been reported in GS to be due to the primary disease process. We present relevant clinical data and hypothesize on why this disease per se may be a risk factor for dilutional hyponatremia.
Keywords
Complication
Gitelman's SYNDROME
hyponatremia
Introduction
Gitelman syndrome (GS) is a rare autosomal recessive disorder caused by mutations in the SLC12A3 gene on chromosome 16q13 encoding the thiazide-sensitive NaCl cotransporter (NCC).[1] Estimated population prevalence is 1:40,000 making it the most common inherited tubulopathy.[1] It is commonly diagnosed in the second or third decade and presents with hypokalemic metabolic alkalosis, hypocalciuria (daily calcium excretion of <2 mg/kg of body weight), hypomagnesemia, and normal blood pressure.[2] Despite lifelong phenomena of renal salt wasting, reports of hyponatremia in this disease is rare and coincident to other commonly attributable causes.[23]
Case Report
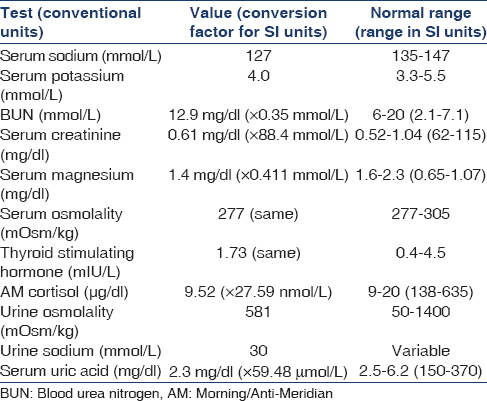
A 60-year-old Caucasian female patient was diagnosed with Gitelman's syndrome when she first presented to our renal clinic in 2000. She had documented a history of persistent hypokalemia and hypomagnesemia since the last 8 years associated with intermittent fatigue and muscle weakness. Past medical history was significant for severe symptomatic hypomagnesemia after an episode of viral gastroenteritis in her early 20s although no detailed work up was done then. She has 3 children none of whom have congenital defects. She is a product of nonconsanguineous marriage. Her brother was diagnosed with “Bartter's syndrome” in adolescence although details of the circumstances leading to either his diagnosis nor genetic testing were available. Her only medications were potassium chloride 20 meq p.o. BID and magnesium oxide 400 mg p.o. BID. She was an occasional drinker, ex-smoker quit 20 years prior, and denied using recreational drugs. Biochemical panel supporting a diagnosis of GS is presented in Table 1. Patient refused genetic testing. Subsequent to our first clinic evaluation, oral potassium and magnesium supplementation was increased and her electrolyte abnormalities remained in normal range till the event of interest.

The patient was lost to follow-up in the renal clinic for 4 years till she returned back in July 2008. Interval history was notable for a brief period of hospitalization in January 2008 from lumbar vertebral compression fracture due to a fall. As per records, preoperative biochemistry panel showed a serum Na of 128 mEq/L (last recorded value was normal in 2007). During the hospital stay, serum sodium dipped further postoperatively to a nadir of 122 mEq/L while on saline infusions and nonsteroidal anti-inflammatory drugs (NSAIDs) and improved spontaneously to 130 mEq/L at the time of discharge. She had no symptoms of hyponatremia, and no renal opinion was sought in-patient. Notably, a patient admitted to be drinking large amounts of water (approximately 4–6 L/a day) to “keep the system clean” since the last 1 year or so. She denied any increasing thirst or compulsive drinking, changes in memory, mood or thought process, using psychoactive substances, antidepressants, opiods, or NSAIDs preoperatively.
On examination, supine pulse and blood pressure were 74/min and 94/58 while readings after 2 min of standing were 80/min and 88/58 with no orthostatic symptoms. In addition, physical examination showed no jugular venous distension, clinical euvolemia and normal chest, cardiac, neurological, and psychiatric evaluation. Repeat biochemical evaluation from our clinic, which are shown in Table 2, in the context of euvolemic hyponatremia and normal thyroid and adrenal function suggested a diagnosis of a syndrome of inappropriate antidiuretic hormone (SIADH) like state. No obvious cause of antidiuretic hormone (ADH) hypersecretion could be discerned in her clinical picture prior to the vertebral fracture. A whole body computed tomogrpahy scan was also done to rule out pulmonary or intracranial lesions as potential sources of ADH hypersecretion and was negative. She was started on fluid restriction to 1.5 L a day. Within 1-month, serum sodium went up to 134 and subsequently she became normonatremic [Figure 1].

- Trends in serum sodium of our patient over the period of clinic follow-up
Discussion
Hyponatremia is a rarely reported complication of GS despite a life-long propensity for renal salt wasting. An extensive literature search revealed only 3 cases of GS reported with coincidental hyponatremia.[23] Schepkens et al. reported 2 cases both with a long-standing diagnosis of GS preceding hyponatremia.[2] The diagnosis in the first case was psychogenic polydipsia which responded to antipsychotic medication to control compulsive free water intake and free water restriction while in the second case, patient had obstructive jaundice secondary to carcinoma of the pancreas.[2] Hyponatremia in the second case was attributed to salt-wasting nephropathy arising from tubule-toxic effects of bile salts in a biliary obstruction which resolved after biliary stenting.[2] In the second paper, Ali et al. reported euvolemic hyponatremia in a 17-year-old boy hospitalized for pneumonia who also had normal blood pressure, hypokalemia, hypomagnesemia, and metabolic alkalosis.[3] A de novo diagnosis of GS was made in the acute setting. The cause of hyponatremia was attributed to SIADH although no hypouricemia was documented and follow-up clinical data specifically related to serum sodium and treatment of hyponatremia was lacking.[3]
The issue of whether the association between hyponatremia in GS is causal or not in our case will remain unsettled although we attempted to exclude all known causes. Dilutional hyponatremia as a direct consequence of a dysfunctional distal tubular NCC protein (due to SCL12A3 mutations in GS) is certainly biologically plausible given the frequent occurrence of hyponatremia from pharmacological blockade of this channel by thiazides. Striking, however, is the unpredictability of thiazide-induced hyponatremia (TIH) suggesting an idiosyncratic mechanism.[45] The propensity of TIH to affect elderly, females and frail individuals along with clinical euvolemia, a low blood urea nitrogen and hypouricemia resulting from increased fractional urate excretion suggest an SIADH like state.[6]
Mechanistically, an SIADH like state with NCC blockage is possible when there is an impaired renal tubular dilutional capacity due to failure to lower distal tubular Na and Cl concentration in the setting of an intact urinary concentrating mechanism.[45678] In addition, net urinary Na loss is high leading to extracellular volume contraction which reduces GFR and promotes vasopressin release leading to further reduction in free water clearance in patients on thiazide diuretics.[89] Direct effects of thiazides in causing TIH such as increased thirst response or increased aquaporin-2 expression are unlikely to be operational in GS and may account for the apparent rarity of hyponatremia in this genetic disease.[57]
Clearance studies on GS patients have demonstrated a decreased free water clearance thereby establishing a potentially favorable setting for hyponatremia in this disease even in the absence of other coexistent and unrelated causes.[10] In this backdrop, a second hit such as a voluntary increase in free water intake (first case of Schepkens et al.[2] and our patient), renal tubular salt wasting (second case of Schepkens et al.[2]) and pneumonia induced ADH hypersecretion (the case of Ali et al.[3]) is sufficient to trigger clinical hyponatremia. The closest differential diagnosis in our patient was reset osmostat syndrome which is usually mild (unlike our case) and does not normalize with free water restriction.[11] Other options for treatment of SIADH such as salt tablets or oral vasopressin receptor antagonist tolvaptan were not considered in our case given the mild and easily correctable nature of hyponatremia.[121314]
To conclude, hyponatremia is a rarely reported but a clinically plausible complication of Gitelman's syndrome resulting from a complex interplay of life-long tubular salt wasting, extracellular volume depletion induced ADH hypersecretion and impaired distal tubular diluting mechanism. Closer studies in index cases of GS reported worldwide can potentially unravel a new risk factor for dilutional hyponatremia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Severe hyponatraemia and hypouricaemia in Gitelman's syndrome. Nephrol Dial Transplant. 2001;16:2250-2.
- [Google Scholar]
- A case of Gitelman syndrome with severe hyponatraemia and hypophosphataemia. Singapore Med J. 2013;54:e18-20.
- [Google Scholar]
- Thiazide-induced hyponatremia. Reproducibility by single dose rechallenge and an analysis of pathogenesis. Ann Intern Med. 1989;110:24-30.
- [Google Scholar]
- Significance of the measurement of uric acid fractional clearance in diuretic induced hyponatraemia. Postgrad Med J. 1986;62:449-52.
- [Google Scholar]
- Thiazide induces water absorption in the inner medullary collecting duct of normal and Brattleboro rats. Am J Physiol. 1999;277(5 Pt 2):F756-60.
- [Google Scholar]
- Diuretic-induced hyponatremia and sustained antidiuresis. Am J Med. 1962;33:783-91.
- [Google Scholar]
- Localization of tubular adaptation to renal sodium loss in Gitelman syndrome. Clin J Am Soc Nephrol. 2012;7:472-8.
- [Google Scholar]
- Hyponatremia due to reset osmostat in dementia with lewy bodies. J Am Geriatr Soc. 2008;56:567-9.
- [Google Scholar]
- A case of persistent hyponatraemia due to reset osmostat. Med J Malaysia. 2006;61:638-40.
- [Google Scholar]
- Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164:725-32.
- [Google Scholar]







