Translate this page into:
A Boy with End-Stage Kidney Disease and Hypertriglyceridemia
Corresponding author: Dr. Sidharth K. Sethi, Pediatric Nephrology, Kidney Institute, Medanta, The Medicity, Gurgaon - 122 001, Haryana, India. E-mail: sidsdoc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Raina M, Savita S, Singh K, Sethi SK. A Boy with End-Stage Kidney Disease and Hypertriglyceridemia. Indian J Nephrol. 2024;34:261–2. doi: 10.4103/ijn.ijn_108_23
Abstract
Mutations in the HNF-1β gene have been found to be associated with renal cysts and diabetes syndrome (RCAD), also known as MODY5. The mutation is inherited in an autosomal dominant fashion, although sporadic mutations can be seen. Pediatric cases of HNF - 1β mutations are more likely to present with renal involvement like renal failure or renal hypoplasia. In young individuals, the detection of renal abnormalities usually pre-date the diagnosis of diabetes with a mean age of 24 years. We report a 5 year old, end stage kidney disease patient with renal cysts and hypertriglyceridemia (in the absence of overt diabetes) with a known pathogenic mutation in the Hepatocyte Nuclear Factor-1β (HNF-1β) gene on chromosome 17q12. This case expands the clinical spectrum of HNF-1β mutation disorders with a take home message, that end stage renal disease patients with unexplained hypertriglyceridemia (even in absence of diabetes mellitus) should alert a clinician for HNF-1β mutational analysis.
Keywords
Renal cysts
HNF
diabetes
MODY
Introduction
The unique mutation in the hepatocyte nuclear factor-1 β (HNF-1β) gene has been found to be associated with renal cysts and diabetes syndrome (RCAD), more commonly known as Maturity-onset diabetes of the young type 5 (MODY5). To date, more than 400 mutations of the HNF1B gene have been identified in RCAD patients, and de novo mutations are encountered in up to 30–50% of cases.1 MODY is characterized by an early age of onset of diabetes and pancreatic β-cell dysfunction. Over 13 different genes have been associated with this disease.1 Overall, <1% of MODY is due to HNF-1β gene mutations,1 inherited in an autosomal dominant pattern or sporadic.2,3 MODY encompasses a large clinical spectrum from isolated diabetes, isolated renal cysts, kidney failure, hyperglycemic hyperosmolar state, and pathologies of the liver and genital tract.4 Here we report a 5-year-old end-stage kidney disease patient with renal cysts and hypertriglyceridemia as a manifestation of a known pathogenic HNF-1β mutation in the absence of overt diabetes.
Case Report
A 5-year-old boy presented with complaints of progressively increasing swelling over the face and abdomen for the last 1 month, with generalized weakness and decreased appetite. The parents also gave a history of the need for blood transfusion once. He had polyuria and polydipsia since early childhood, with failure to thrive and delayed gross motor milestones. His clinical examination showed a weight of 12 kg (less than third percentile) and a height of 98 cm (less than third percentile). There was no evidence of rickets on examination. Laboratory investigations are given in Table 1, and renal ultrasound is shown in Figure 1. In view of severe metabolic acidosis and uremia, the patient was initiated on maintenance hemodialysis.
| Laboratory parameter | Laboratory value | Normal value |
|---|---|---|
| Hemoglobin | 8.4 mg/dL | 11–14 g/dL |
| Serum calcium | 7.8 mg/dL | 8.4–10.2 mg/dL |
| Serum phosphorous | 8.7 mg/dL | 2.5–4.5 mg/dL |
| Blood urea | 168 mg/dL | 19–40 mg/dL |
| Serum creatinine | 6.4 mg/dL | 0.5–0.8 mg/dL |
| Serum uric acid | 4.5 mg/dL | 3–6 mg/dL |
| Serum magnesium | 2.6 mg/dL | 1.6–2.5mg/dL |
| Serum sodium | 148 meq/L | 135–145 meq/L |
| Serum potassium | 3 meq/L | 3.5–5.5 meq/L |
| Fasting blood glucose | 110 mg/dL | <100 mg/dL |
| Parathyroid hormone | 164.5 pg/mL | 15–70 pg/mL |
| Lipid profile | ||
| Total cholesterol | 478 mg/dL | <200 mg/dL |
| LDL cholesterol | 211 mg/dL | <130 mg/dL |
| HDL cholesterol | 50 mg/dL | 40–60 mg/dL |
| VLDL cholesterol | 217 mg/dL | <28 mg/dL |
| Triglycerides | 481 mg/dL | <150 mg/dL |
| Ultrasound | Both kidneys showed increased echogenicity with obliterated corticomedullary differentiation and multiple tiny cortical cysts. | |
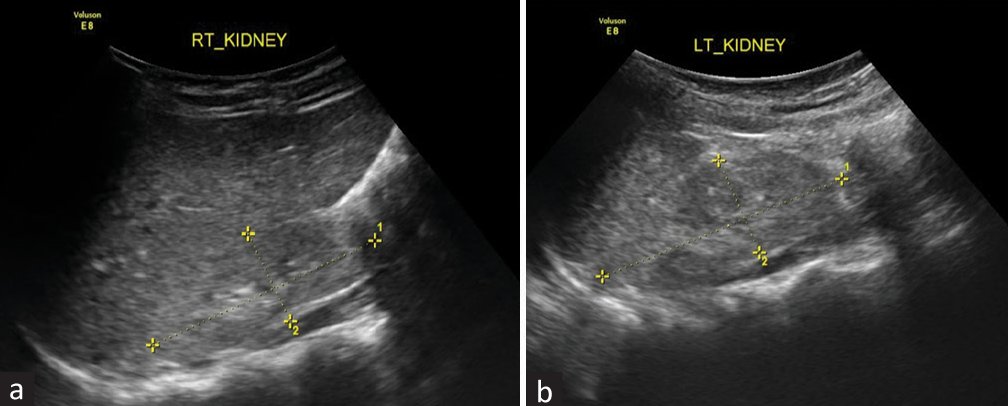
- (a and b) Both kidneys showed increased echogenicity with obliterated corticomedullary differentiation and multiple tiny cortical cysts.
Whole exome sequencing showed heterozygous missense mutation on codon 716 of exon 3 [c.716G> A (p.Glycine239GlutamicAcid)] on HNF-1β gene on chromosome 17q12 (HNF1B [ENST00000617811.5], c. 716G>A, p.Gly239Glu). This variant was classified as pathogenic using ACMG criteria (PM1 + PM2 + PM5 + PP3 + PP5). This variant has not been found in healthy population databases such as 1000 genomes, GnomAD including the South Asian population.
The parents of the proband were tested and did not carry the same variant, hence it was a de novo mutation in our patient. Both parents’ blood urea, serum creatinine, uric acid, fasting blood glucose, HBA1C, and ultrasound abdomen were normal. A similar mutation has been reported in China in an 11-year-old girl, with renal cysts and hyperglycemia hyperosmolar coma.4 At a follow-up of 1 year, the child is on maintenance hemodialysis, and has normal glucose control (HBA1C 4.2%; fasting blood glucose 87 mg/dL).
Discussion
The normal kidney development process requires HNF-1β at multiple steps, as shown by gene targeting studies.3 Pediatric cases of HNF-1β mutations are more likely to present with renal involvement like renal failure or renal hypoplasia.5 Other associated renal anomalies include multicystic renal dysplasia, renal hypoplasia, unilateral renal agenesis, microcystic dysplasia, horseshoe kidney, duplicated ureters, atypical familial juvenile hyperuricemic nephropathy, and urinary tract malformations.1,6 In a study, approximately 13– 15% of patients developed end-stage renal disease.1
Our patient was unique since the child presented with chronic kidney disease stage 5, renal cysts, and dyslipidemia without overt diabetes. In a study of nine patients who were carriers of HNF-1β mutation, eight had hyperlipidemia.7 In the study, 45% of patients were diagnosed secondary to renal disease and did not initially present with diabetes.7 There are previous reports also of isolated hypertriglyceridemia in the absence of poor glycemic control or obesity in an adult female with 17q12 HNF-1β mutation, which may suggest hypertriglyceridemia as a feature of HNF-1β-related functional defect.8 The renal abnormalities usually pre-date the diagnosis of diabetes in young patients with a mean age of 24 years.8 Severe dyslipidemia, renal cysts, and the presence of a known pathogenic mutation clinched the diagnosis in our case.
No specific therapy is available, however diagnosing the disease in a timely manner helps the family seek screening for diabetes, renal function decline, hypomagnesemia, and associated hypokalemia. It is important to screen for diabetes and start treatment early.8 Moreover, additionally, unnecessary biopsies and tests may be avoided.1
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Hepatocyte nuclear factor 1ß-associated kidney disease: More than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345-53.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatocyte nuclear factor 1ß (HNF1ß)-associated disease | Genetic and Rare Diseases Information Center (GARD) - An NCATS Program. Available from: https://rarediseases.info.nih.gov/diseases/13702/hepatocyte-nuclear-factor-1-hnf1-associated-disease [Last accessed on 2023 Apr 28]
- [Google Scholar]
- Multiomics analysis reveals that hepatocyte nuclear factor 1β regulates axon guidance genes in the developing mouse kidney. Sci Rep. 2022;12:17586.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatocyte nuclear factor 1β maturity-onset diabetes of the young in a Chinese child presenting with hyperglycemic hyperosmolar state. Acta Diabetol. 2017;54:969-73.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: Results of the ESCAPE study. J Am Soc Nephrol. 2006;17:2864-70.
- [CrossRef] [PubMed] [Google Scholar]
- The prenatal and postnatal incidence of congenital anomalies of the kidneys and urinary tract (CAKUT) detected by ultrasound. Child Kidney Dis. 2016;20:29-32.
- [CrossRef] [Google Scholar]
- 16-OR: Endocrine manifestations of pediatric HNF1B-MODY (MODY 5) Diabetes. 2021;70(Suppl 1) doi: 10.2337/db21-16-OR
- [CrossRef] [Google Scholar]
- Hypertriglyceridemia as a main feature associated with 17q12 deletion syndrome-related hepatocyte nuclear factor 1ß-maturity-onset diabetes of the young. Endocrinol Diabetes Metab Case Rep. 2022;2022:22-0297. doi: 10.1530/EDM-22-0297
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







