Translate this page into:
A case of fatal disseminated Apophysomyces elegans infection in a renal allograft recipient
Address for correspondence: Dr. B. Pawar, Renal Clinician Alice Springs Hospital, Alice Springs, NT 0871, Australia. E-mail: basant.pawar@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
An unusual fatal infection with Apophysomyces elegans belonging to the fungal class Zygomycetes in a renal transplant recipient is presented.
Keywords
Apophysomyces elegans
fungal infection
renal transplantation
Zygomycetes
Introduction
Fungal class Zygomycetes of the order Mucorales are abundant in the soil. The morphological features of this fungus in infected tissue demonstrate broad, non-septate hypha with right-angled branching. The hypha of Mucorales often invade the walls and lumen of blood vessels[12] with a predisposition to intracerebral vessels[3] resulting in thrombosis and infarction of surrounding tissue. Zygomycosis occurs in immunocompetent[4] as well as in immunodeficient patients including in organ transplant recipients, those with malignancies and in diabetics. Apophysomyces elegans is a fungus belonging to this class that characteristically produces post-traumatic locally invasive cutaneous infection.[5] The most frequent reported form of presentation is rhino cerebral followed by pulmonary, gastro intestinal, cutaneous, subcutanueous, and occasionally disseminated infection.[36] An increased incidence and mortality from invasive mycosis have been seen in transplant recipients.[78] A case of disseminated A. elegans infection in an Australian indigenous’ deceased donor renal transplant recipient is reported.
Case Report
A 55-year-old Australian indigenous male, with chronic kidney disease of uncertain etiology, received a deceased donor renal transplant in September 2008. Prior to renal transplantation, he was treated for latent tuberculosis. His immunosuppression included induction with basiliximab followed by maintenance with tacrolimus, mycophenolate mofetil and prednisolone. There was no history of foreign travel.
Eight months after transplantation, he presented with 2 days history of a well circumscribed discolored lesion on the anterior abdominal wall and the left thigh, following insect bites while camping in the “bush”.
He was afebrile vital signs were normal. An indurated, well demarcated, hyperpigmented tender lesion, 4 cm × 4 cm was present on the right anterior abdominal wall and another lesion 10 cm × 3 cm on the anterior aspect of his left thigh. He also had a tender swelling on the posterior aspect of the right calf. The lesions over the anterior abdominal wall and the left thigh increased in size with the breakdown and necrosis of the overlying skin. Neurological examination revealed Grade 4 power in the left leg with reduced pin prick in L2/3/4 dermatomal distribution with absent left knee and ankle reflexes. Plantar response was equivocal on the left foot.
His investigations were – total leukocyte count 3.7 × 109/L, neutrophils 2.5 × 109/L, lymphocytes 0.4 × 109/L, hemoglobin 12.9 g/dl, platelet count 1.53 lakhs/mm3, creatinine kinase 722 U/L, serum 2.5 mg/dl, C-reactive protein 209 mg/L, bilirubin 0.52 mg/dl, alkaline phosphatase 314 U/L, alanine aminotransferase 22 U/L, Gammaglutamul transpeptidase 34 U/L, serum albumin 41 g/L, total protein of 7.8 g/dl Chest X-ray showed a small right lower lobe consolidation. Hepatitis A, B and C antibodies were not detected. Q fever, rickettsial serology and Cryptococcus neoformans antigen negative in the serum. Urine and blood cultures were sterile.
In the hospital, he developed intermittent fever up to 39.5°C. He was empirically commenced on intravenous cephazolin for presumed cellulitis after blood and urine cultures were drawn.
Ultrasound examination showed edema of the subcutaneous tissues, but no focal collections. Skin biopsies of the abdominal and right calf lesions were obtained. Initial gram stain of the biopsy specimen did not show any organism. Subsequent urine and blood cultures were sterile. Computed tomography (CT) scan of the thorax [Figure 1], abdomen and lumbar spine showed an inflammatory mass lesion within the right lower lobe lung with edema of the left iliopsoas muscle.
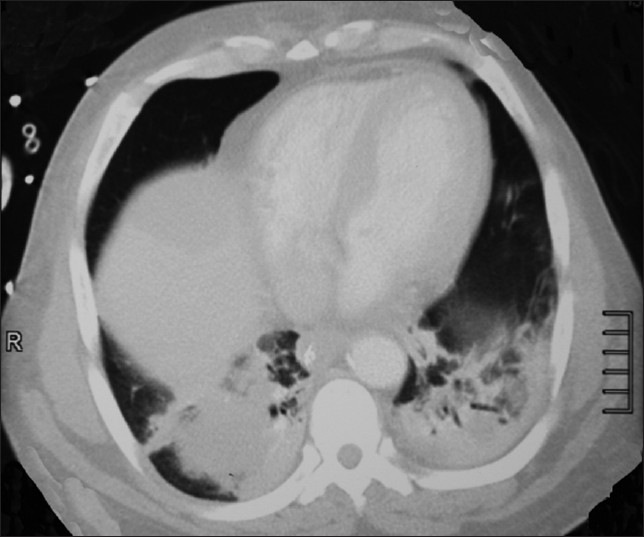
- Bilateral lower lobe consolidation
Four days after presentation, he became confused and restless. He developed hypotension. In the intensive care unit, he was initiated on inotropes, was intubated and ventilated. Meropenem, doxycycline, vancomycin and intravenous voriconazole were administered.
A whole body CT scan was carried out, which showed a large left occipital infarct with hemorrhagic transformation, [Figure 2], and wide spread infarctions in the spleen, liver, native kidneys, and transplanted kidney. These were presumed to be secondary to the spread of the fungal infection. Echocardiographic examination was normal.
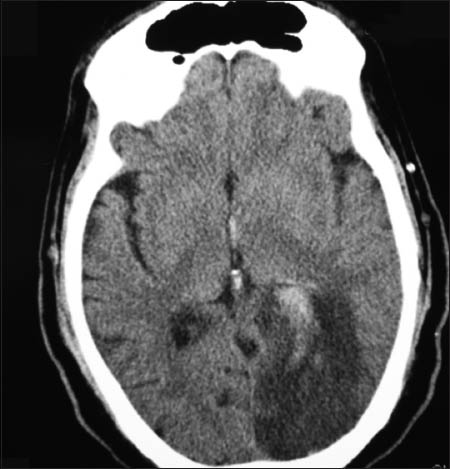
- Left sided occipital cerebral infarction
The patient's general condition continued to deteriorate, and he died 6 days after presentation. An autopsy was not performed.
Fungal cultures obtained from skin biopsies, subsequently showed growth of a zygomycete, identified as A. elegans.
Discussion
Infections due to A. elegans are often opportunistic in nature and are seen in both immunocompetent as well as in immunocompromised individuals. Originally isolated from mango orchard soil in India, A. elegans was described as a new genus and species of mucorales by Misra et al. in 1979.[9] The disease when occurs in the upper and lower airways, is associated with sinusitis, rhino cerebral mucormycosis, or pulmonary infection. Rhino cerebral mucormycosis occurs primarily in patients with diabetic ketoacidosis but has also been described in patients with neutropenia and in renal transplant recipients.[10] In an Indian study of fungal infections of the class zygomyecetes of the order mucorales, rhinocerebral presentation constituted 44.2%, cutaneous 15.5% and renal in 14% of all cases.[6] Hemodialysis patients with iron overload, who have received desferoxamine are at particular risk for rhino cerebral and disseminated mucormycosis.[11] A. elegans infections however are not always limited to the immunosuppressed host, and several cases have been reported in those previously immunocompetent.[4612] A. elegans infection has been described following traumatic inoculation from soil contamination,[13] including following burns.[14] Once established, the infection disseminates to various internal organs. In all disease forms, vascular invasion by hypha is predominant with ischemic and hemorrhagic necrosis being prominent histological findings.[12]
Identifying the exact species of the fungus is often difficult. Morphology of A. elegans in culture is similar to that of Absidia species. It also has a foot cell that resembles those produced by aspergillus species. However, only A. elegans has what has been described as a dark brown campanulate or funnel shaped apophyses with an exceptionally dark pigmented thick walled zone of the sporangiophore just below the apophyses.[1516]
The possibility of unusual fungal infections should be considered in the setting of continued clinical deterioration despite adequate antibacterial therapy. A high index of suspicion and obtaining early tissue biopsies and cultures is an important. Successful treatment requires early diagnosis and removal of infected tissues (histological examination is vital in deciding the extent of debridement that will be necessary) along with appropriate systemic antifungal therapy. Liposomal amphotericin B still remains the drug of choice for A. elegans infection.[612]
In this patient, the lesions appeared after trivial insect bites, followed by rapid dissemination of the fungus throughout the body resulting in his demise.
Review of the literature indicates that even when early vigilance and aggressive therapy are applied, the mortality remains high for systemic zygomycosis.[468]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Phycomycosis. A clinicopathologic study of fifty-one cases. Lab Invest. 1962;11:963-85.
- [Google Scholar]
- Case 48-1968. Facial pain, ophthalmoplegia and obtundation with hyperglycemia and uremia. N Engl J Med. 1968;279:1220-29.
- [Google Scholar]
- The computed tomographic spectrum of intracranial mycosis: Correlation with histopathology. Radiology. 1981;141:703-7.
- [Google Scholar]
- Apophysomyces elegans: An emerging zygomycete in India. J Clin Microbiol. 2003;41:783-8.
- [Google Scholar]
- Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect. 2001;42:261-6.
- [Google Scholar]
- Drugs used in prophylaxis and treatment of fungal infections in immunosuppressed children. Przegl Lek. 2004;61(Suppl 2):89-94.
- [Google Scholar]
- Two decades of experience in mucormycosis after kidney transplantation. Ann Transplant. 2011;16:44-8.
- [Google Scholar]
- Rhinocerebral mucormycosis in renal transplant recipients: Report of three cases and review of the literature. Rev Infect Dis. 1986;8:441-6.
- [Google Scholar]
- Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin Infect Dis. 1993;17:881-4.
- [Google Scholar]
- Apophysomyces elegans as an agent of zygomycosis in a patient following trauma. J Med Vet Mycol. 1992;30:83-6.
- [Google Scholar]
- Burn wound zygomycosis caused by Apophysomyces elegans. J Clin Microbiol. 1990;28:2151-3.
- [Google Scholar]
- Mucormycosis. In: Kwon-Chung KJ, Bennett JE, eds. Medical Mycology. Philadelphia: Lea and Febiger; 1992. p. :524-59.
- [Google Scholar]
- Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J Clin Microbiol. 1988;26:1861-3.
- [Google Scholar]







