Translate this page into:
A Prospective Study of Pulmonary Hypertension in Patients with Chronic Kidney Disease: A New and Pernicious Complication
Address for correspondence: Dr. B. S. Arun, Department of General Medicine, MVJ Medical College, Bengaluru - 562 114, Karnataka, India. E-mail: arunbsmedicine@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pulmonary hypertension (PH) is a recently recognized complication of chronic kidney disease (CKD), especially in end-stage renal disease. It has prevalence estimates of 30%–50% and is an independent predictor of increased mortality in CKD patients. The aim of this study is to analyze the prevalence of PH in patients with CKD, its severity in different stages of CKD, and risk factors for it. One hundred and eight patients with CKD treated at Karnataka Institute of Medical Sciences, Hubli, Karnataka, between January 1, 2014, and June 30, 2015, were selected. Clinical evaluation and relevant investigations including echocardiography were done. Follow-up echocardiography was done at 3 and 6 months and assessed. The mean age of studied population was 43.53 ± 14.63 years. Sex ratio was 2.72:1 (male:female). PH was present in 47 of 108 (43.5%) cases at beginning, 41 of 83 (491.4%) at 3 months, and 32 of 64 (50%) at 6 months. The prevalence and severity of PH increased with progression of CKD stage, although not statistically significant. Heart failure with reduced ejection fraction and heart failure with preserved EF were significantly higher among PH group compared to non-PH group (P < 0.01). Mean hemoglobin in PH group was significantly lower, compared to non-PH group (P < 0.01). Mean interdialytic weight gain and central venous pressure were higher among PH group than non-PH group. Higher calcium phosphate product ≥50 was more prevalent in PH group than in non-PH group. The majority of them had moderate PH at the beginning of the study which remained same, despite being on hemodialysis. PH is a common complication in CKD patients with prevalence of 43.5%–50%. Left-sided heart failure, anemia, fluid retention, and increased calcium phosphate product are the risk factors for developing PH.
Keywords
Calcium phosphate product
chronic kidney disease
ejection fraction
pulmonary hypertension
Introduction
Chronic kidney disease (CKD) is a common health problem worldwide. Cardiovascular disease is the most common cause of morbidity and mortality in CKD.[1] Pulmonary hypertension (PH) is an overlooked cardiovascular complication of CKD, especially in end-stage renal disease (ESRD). The prevalence of PH in patients with ESRD ranges from 27% to 58%.[2] Patients with advanced CKD have a prevalence of PH that is lesser than that in patients with ESRD, ranging from 8% to 39%.[3456] PH is an independent predictor of increased mortality in patients with CKD.[23]
PH is defined as “a mean pulmonary artery pressure more than or equal to 25 mmHg at rest or 30 mmHg at exercise.” The pathogenesis of PH in CKD is not fully elucidated.[2] It is considered to be due to interaction of multiple aspects of altered cardiovascular physiology. Myocardial dysfunction leading to elevated left ventricular filling pressure and pulmonary venous hypertension is the predominant cause of PH in CKD.[367] The other factors implicated are increased cardiac output (CO),[23] increased pulmonary blood flow due to shunting across arteriovenous fistula (AVF)[37] volume overload, anemia, exposure to dialysis membranes,[7] endothelial dysfunction leading to pulmonary vasoconstriction, decreased compliance of pulmonary vasculature, vascular calcification and stiffening,[7] increased thromboxane B2, and pro-brain natriuretic peptide.[4] This study was conducted to assess prevalence, severity and risk factors of PH in CKD.
Methods
Source of data
One hundred and eight patients with CKD treated at General Medicine, Nephrology and Cardiology wards at Karnataka Institute of Medical Sciences (KIMS), a government-run tertiary care hospital, Hubli, Karnataka, between January 1, 2014, and July 31, 2015, were considered for the study.
Sample size calculation
The sample size was estimated using the proportion of PH in CKD patients from the literature as 50%. Using the formula,
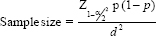
Here,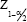 = Is standard normal variate (at 5% type 1 error [P < 0.05], it is 1.96; at 1% type 1 error [P < 0.01], it is 2.58). As in majority of studies, P < 0.05 was considered statistically significant and hence 1.96 is used in the formula.
= Is standard normal variate (at 5% type 1 error [P < 0.05], it is 1.96; at 1% type 1 error [P < 0.01], it is 2.58). As in majority of studies, P < 0.05 was considered statistically significant and hence 1.96 is used in the formula.
p = Expected proportion in population based on previous studies or pilot studies.
d = Absolute error or precision – has to be decided by researcher.
P = 50; q = 50; d = 10%.
Using the above values at 95% confidence level, a sample size of 98 patients with CKD was need to be included in the study. Considering 10% non-response, a sample size of 98 + 9.8 ≈ 108 individuals were included in the study.
One hundred and twenty-five consecutive patients with CKD were evaluated for inclusion and exclusion criteria, of which 108 patients fulfilling the criteria were considered for the study. This study complies with the Declaration of Helsinki. Clearance was obtained from the Ethical Committee of KIMS, Hubli, Karnataka.
Methods of collections of data
-
Selected patients were informed about objectives of the study, and informed consent was taken from the patient or their guardian
-
Information was collected using a preformed proforma from each patient.
Type of study
This was a prospective observational study.
Inclusion criteria
-
Newly diagnosed cases of CKD based on Kidney Disease Improving Global Outcome 2012 criteria, including CKD patients on hemodialysis
-
Age ≥18 years.
Exclusion criteria
-
Those not willing to participate in the study
-
Age <18 years
-
Valvular heart diseases
-
Congenital heart diseases
-
Pulmonary obstructive and restrictive diseases
-
HIV-infected patients
-
Chronic liver disease
-
Connective tissue diseases
-
Hypothyroidism and hyperthyroidism.
Protocol
The history was elicited with special reference to symptoms of CKD, congestive heart failure, PH, risk factor for developing CKD, comorbid conditions, duration of diagnosis of CKD, and HD. Clinical signs of CKD, heart failure, and PH were assessed. Each case underwent relevant investigations. Staging of CKD was done based on estimated glomerular filtration rate (eGFR) estimated by Modification of Diet in Renal Disease formula. Electrocardiogram (ECG) was done to look for features of PH, right ventricular (RV) strain pattern, left ventricular (LV) strain pattern, and IHD.
Echocardiography was done in all patients at the beginning of the study, 3 months, and 6 months. Pulmonary artery systolic pressure (PASP) was calculated using TR jet velocity in Doppler echocardiography and applying Bernoulli equation. A PASP value of ≥35 mmHg at rest was taken to be suggestive of PH. Echocardiography was also used to assess the LV hypertrophy, dilatation, chamber size, LV systolic function and diastolic function, LV ejection fraction (EF), regional wall motion abnormality, and pericardial effusion. Comparison was made between CKD patients with presence and absence of PH.
Statistical analysis
Descriptive and inferential statistical analysis had been carried out in the study. The continuous variables such as age, blood pressure (BP), and EF were expressed in terms of mean ± standard deviation (SD). The categorical measurements were expressed in number (percentage). Significance was assessed at 5% level of significance (P < 0.05).
Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups with presence and absence of PH (intergroup analysis) on metric parameters. Chi-square/Fisher's exact test has been used to find the significance of study parameters on categorical scale between two or more groups.
Statistical software
The statistical software, namely, SAS 9.2 (SAS institute Inc. 2010 Cary, North Carolina, USA) andSPSS 20 (version 20.0, IBM Corp. 2010 Armonk, NY, USA), were used for the analysis of the data, and Microsoft word and Excel were used to generate graphs, tables, etc.
Results
General characteristics
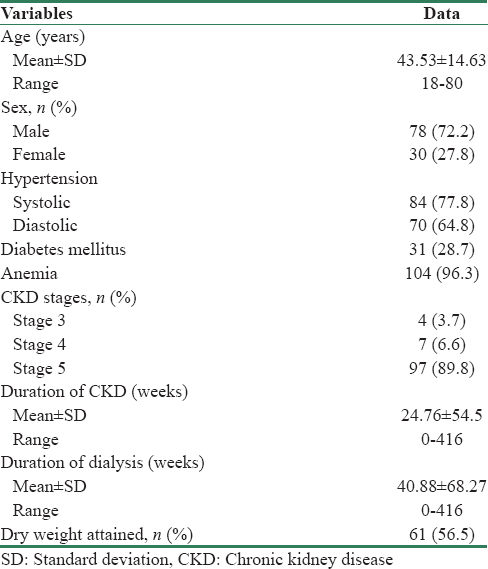
The mean age of the study population was 44.53 ± 14.63 years. The majority were of age group of 31–50 years (67.6%), which represent the productive age group of the society. Sex ratio was 2.6:1 [Table 1]. Overview of study results are depicted in [Figure 1].
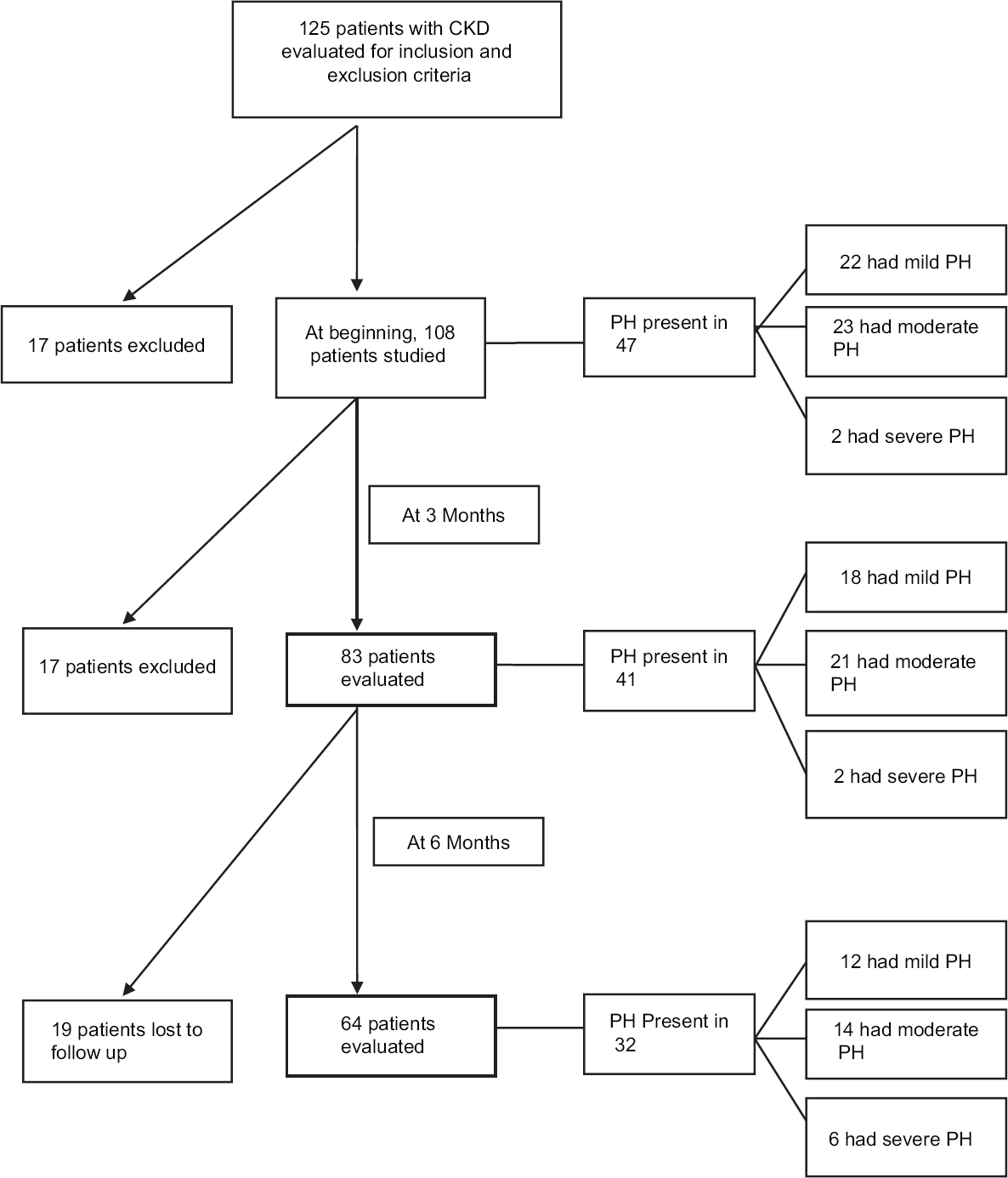
- Flowchart showing key results of the study
Echocardiographic findings
Assessment of pulmonary hypertension
Of 108 patients who were assessed with ECHO at beginning of the study, 47 (43.5%) patients had PH. The mean PASP among patients with PH at this ECHO was 51.1 ± 13.3 mmHg. The majority had moderate PH.
After 3 months, ECHO could be done in 83 patients, of which 41 (49.4%) had PH. Rest of the patients either expired or discontinued follow-up. Among the patients with PH, the mean PASP was 51.46 ± 10.31 mmHg. The majority had moderate PH. Then, at further follow-up at 6 months, ECHO was done in 64 patients, of which 32 (50%) had PH. The mean PASP among patients with PH was 55.44 ± 14.41. The majority had moderate PH. The percentage prevalence of PH increased as the time elapsed. The majority of the patients had moderate PH, throughout the study [Table 2].

Among the patients with PH, right ventricular and right atrial dilatation was present in 42 of 47 (89.4%) patients in first ECHO, 36 of 41 (87.8%) patients in ECHO done at 3 months, and 29 of 32 (90.6%) patients in ECHO done at 6 months. All patients with right ventricular and right atrial dilatation had PH.
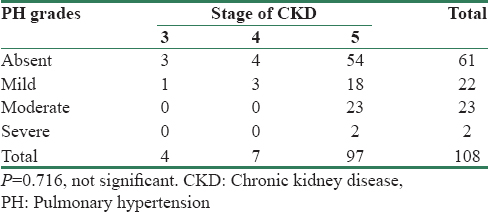
Among patients with CKD stage 3 and 4, majority had mild PH, but in stage 5, it was predominantly moderate PH (23 of 97 [23.7%]) patients. This indicates that PH increased in severity with progression of CKD [Table 3], although this was not statistically significant, since there were few patients in stage 3 and stage 4.
Among 108 patients, 57 were on thrice-weekly HD and 51 were on twice-weekly HD. Of these 57 patients on thrice-weekly HD, 24 had PH (42.1%). Of 51 on twice-weekly HD, 23 had PH (45%). There was no significant difference in prevalence of PH between these 2 groups (P = 0.85).
Assessment of other echocardiographic findings
Heart failure with reduced EF (HFrEF) was present in 29 (26.6%) of 108 patients. Among the patients with LV systolic dysfunction, majority had mild dysfunction [Table 2].
The prevalence of heart failure with preserved EF (HFpEF,) was 95 (85.2%) of 108 patients at first ECHO, increased further at follow-up ECHO [Table 2].
Comparison between the groups with presence and absence of pulmonary hypertension
The prevalence of clinical features of PH dyspnea, loud P2, and ascites were significantly higher among the patients with PH compared to those without PH [Table 4].

There was no significant difference in various ECG findings between the patients with PH and without PH [Table 4]. HFrEF, HFpEF, and pericardial effusion were significantly higher among the patients with PH, compared to those without PH [Table 4].
Mean ± SD of central venous pressure (CVP) and interdialytic weight gain among the patients with PH was significantly higher compared to those without PH (P = 0.05 and P = 0.014, respectively). This implies that volume overload is associated with increased risk of PH. Dialysis duration was higher among the patients with PH than those without PH (P = 0.09) [Table 5].

Mean ± SD of hemoglobin (Hb) and EF was significantly lower among the patients with PH compared to those without PH. This implies that anemia and LV failure are associated with increased risk of PH [Table 5].
Outcome difference between the two groups
The mortality rate in our study population by the end of 1.5 years was 24.1% (26 of 108). It was about two times higher among the patients with PH compared to those without PH (P = 0.03) [Figure 2].
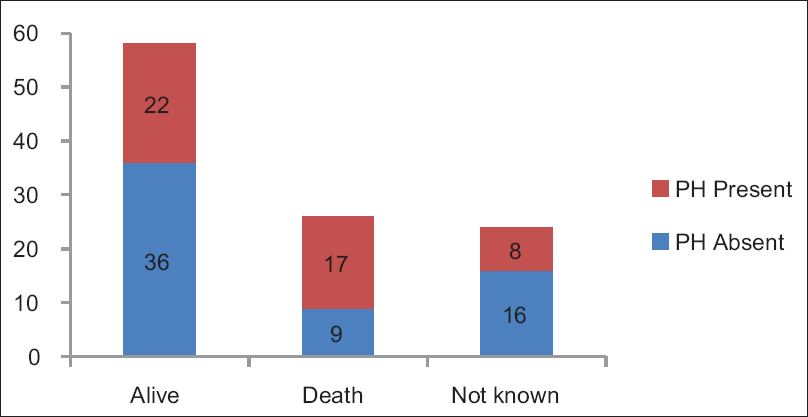
- Outcome of the patients at the end of the study at 1.5 years
Discussion
PH remained an underestimated issue in CKD patients until the very recent years. In our study, majority of the patients in all the three echocardiography had moderate PH. This is consistent with other studies.
PH in CKD can occur through multiple mechanisms, including PH of WHO Group 1–5 (pulmonary arterial hypertension, left-sided heart disease, chronic pulmonary disease and hypoxia, chronic thromboembolic disease, and unexplained PH, respectively).[2] Statistically significant risk factors for developing PH found in our study population were as follows:
-
HFrEF (LV systolic failure) HFpEF (LV diastolic failure), Anemia (P = 0.003)
-
Calcium × phosphate product ≥50 Higher interdialytic weight gain, indicating fluid retention and volume overload (P = 0.014)
-
“Higher CVP, indicating fluid retention and volume overload.” Role of above factors have been studied in various previous studies as depicted in Table 6.

Role of left ventricular failure in causing pulmonary hypertension in CKD
The prevalence of heart failure in patients with CKD is 30%–40%.[1011] This is the most important mechanism contributing to PH (WHO Group 2) in CKD patients and has been extensively evaluated in many studies.[238] In our study, HFrEF was present in 20.4%. Its prevalence among the patients with and without PH was 38.3% and 6.6%, respectively (P < 0.001). The mean EF among the patients with PH was significantly lower, compared to those without PH. This is consistent with the study by Kumbar et al.[8] and Fabbian et al.[9] HFpEF was present in 85.2% of cases. The prevalence of HFpEF was significantly higher among the patients with PH, compared to those without PH (P = 0.006). This is consistent with the study by Abdelwhab et al.[4]
HFrEF and HFpEF were persistent despite being on HD in our study, thus contributing to the development of elevated LV filling pressure and pulmonary venous hypertension. Although HD corrects volume overload and removes the toxic metabolites, it did not have a significant impact on LV function and PASP.
LV failure is a multifactorial process in patients with CKD. It is caused by chronic volume overload, elevated mean arterial pressure, uremia-mediated cardiac myocyte dysregulation, anemia-mediated hypoxemic stress, and impairment in cardiac function by microvascular and macrovascular coronary artery disease.[12] Hypertension and diabetes mellitus, which are two dominant causes of CKD, trigger LV diastolic dysfunction, an alteration bound to increase pulmonary venous and arterial pressure.[13] However, in our study, there was no significant difference in prevalence of hypertension and diabetes mellitus between the two groups.
Persistent elevation of the PCWP owing to impaired diastolic relaxation leads to vascular remodeling with thickening of the pulmonary capillary endothelial basal lamina and proliferation of connective tissue surrounding the alveoli. A study of 76 ESRD by Abdelwhab et al. showed that patients with diastolic dysfunction have an odds ratio of 21.9 for developing PH.[4]
Both proteinuria and reduction of GFR have been associated with increased cardiovascular morbidity and mortality.[1314] This association is so strong and clinically relevant that the diagnosis of CKD places a patient into the highest cardiovascular risk level, irrespective of stratification according to traditional cardiovascular risk factors.[1415]
The high mortality among CKD patients on renal replacement therapy, which for the ages between 25 and 35 years may rise up to 375-fold compared to the general population, is derived predominantly from cardiovascular causes.[1617] This cardiovascular morbidity occurring in patients with CKD is called cardiorenal syndrome type 4, which reflects tight interlink between the heart and kidney.[18]
Role of other factors in causing pulmonary hypertension in CKD
Lower diastolic BP (DBP) was found to be associated with PH in a study by Kumbar et al.[8] However, in our study, there was no significant difference in either DBP or systolic BP between the patients with and without PH (P = 0.383 and P = 0.659, respectively). Anemia is a common manifestation of CKD and important risk factor for developing PH found in our study. The mean Hb was significantly lower among PH group compared to non-PH group, thus implicating the role of anemia in PH. This is consistent with the study by Yigla et al.[3] and Etemadi et al.[19]
In our study, Percentage prevalence of higher calcium phosphate product was significantly greater among those with PH, compared to those without PH. This is consistent with the study by Kumbar et al.[8] Increased calcium phosphate product predisposes to pulmonary vascular calcification, leading to PH.[28]
In our study, interdialytic weight gain and CVP were significantly higher among the patients with PH, compared to those without PH This is consistent with the study by Fabbian et al.[9] This suggests fluid retention to be a contributing factor for developing PH.
Role of hemodialysis in pulmonary hypertension in chronic kidney disease
In our study, the mean dialysis duration was found to be higher among the patients with PH than those without PH. This is consistent with the study by Fabbian et al.[9] The pathogenic mechanisms proposed for PH in CKD patients on hemodialysis are AVF-induced, increased CO, which cannot be accommodated by pulmonary circulation. In addition, pulmonary vessels show signs of endothelial dysfunction resulting in dysregulation of vascular tone due to an imbalance between vasodilators, such as prostacyclins and nitric oxide (NO), and vasoconstrictors, such as endothelin-1, plasma asymmetric dimethylarginine, and thromboxane B2, and local as well as systemic inflammation. It is also believed that microbubbles escaping from the dialysis circuit can trigger vasoconstriction and vascular sclerosis.[420] There was no significant difference in PH prevalence between those who had thrice-weekly and twice-weekly HD. Whether improving the frequency and quality of HD improves heart failure and hence PH is still an unanswered issue and further studies are needed in this regard.
Outcome difference in CKD patients between the groups with presence and absence of pulmonary hypertension
The mortality rate among those with PH was significantly higher, compared to those without PH (P = 0.03). Mortality was twice among those with PH, compared to those without PH. This is consistent with study by Yigla et al.[4] and Ramasubbu et al.,[21] which also showed higher mortality among those with PH.
Limitations of the study
In our study, sample size was small, AVF flow rate and CO could not be assessed, RV catheterization study could not be performed as most of the patients were not willing.
Conclusion
Our study concludes that substantial number of the patients with CKD develops PH. HFrEF and HFpEF are the strongest risk factors. Hence, PH in CKD is predominantly WHO group 2. Thus, PH can be considered as a reflection of cardiorenal syndrome type 4. The other factors contributing to it are anemia, volume overload, and increased calcium phosphate product. PH may be a cause for refractory dyspnea, edema, and ascites in patients with CKD. Long-standing PH is associated with increased morbidity and mortality. Estimation and follow-up of PASP by Doppler echocardiography may be indicated in all the patients with CKD, especially ESRD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. K.P. Suresh, Ph.D (Biostatistics), Scientist (SS), National Institute of Veterinary Epidemiology and Disease Informatics, Yelahanka, Bengaluru - 560 064, India.
References
- Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. 2013;84:682-92.
- [Google Scholar]
- Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577-82.
- [Google Scholar]
- Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28:990-7.
- [Google Scholar]
- Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74:503-10.
- [Google Scholar]
- Ventricular function in patients with end-stage renal disease starting dialysis therapy: A tissue Doppler imaging study. Echocardiography. 2012;29:1054-9.
- [Google Scholar]
- Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial. 2007;23:127-31.
- [Google Scholar]
- Pulmonary hypertension in dialysis patients: A cross-sectional Italian study. Int J Nephrol. 2010;2011:283475.
- [Google Scholar]
- Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int. 2004;65:2380-9.
- [Google Scholar]
- A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38:992-1000.
- [Google Scholar]
- Blood pressure and not uraemia is the major determinant of arterial stiffness and endothelial dysfunction in patients with chronic kidney disease and minimal co-morbidity. Atherosclerosis. 2011;216:217-25.
- [Google Scholar]
- The metabolic syndrome, diabetes and lung dysfunction. Diabetes Metab. 2008;34:447-54.
- [Google Scholar]
- K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266.
- [Google Scholar]
- Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol. 2006;17:2034-47.
- [Google Scholar]
- Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048-56.
- [Google Scholar]
- Patient Characteristics Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2009
- [Google Scholar]
- Definition and classification of Cardio-Renal Syndromes: Workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1416-20.
- [Google Scholar]
- Unexplained pulmonary hypertension in peritoneal dialysis and hemodialysis patients. Rev Port Pneumol. 2012;18:10-4.
- [Google Scholar]
- A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: Prevalence and clinical significance. Int J Gen Med. 2010;3:279-86.
- [Google Scholar]







