Translate this page into:
A Rare Case of Type 4 Rapidly Progressive Glomerulonephritis (Atypical) with Mesangial IgA Deposits: A Case Report
Address for correspondence: Dr. Puneet Bhuwania, Internal Medicine, DNB Nephrology Resident, Department of Nephrology, KG Hospital, Government Arts College Road, Coimbatore - 641 018, Tamil Nadu, India. E-mail: punit101.pb@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Rapidly progressive glomerulonephritis can result from glomerular deposition of anti-GBM antibody, immune complexes, or may involve pauci-immune mechanisms. The coexistence of IgA nephropathy, anti-GBM, and anti-neutrophilic cytoplasmic antibodies is unheard of, and the pathogenic role of these antibodies in IgA nephropathy or vice versa remains unclear. Herein, we describe a case of a patient with type 4 rapidly progressive glomerulonephritis who was found to have significant mesangial IgA deposits. The prognosis of this remains unclear but our patient responded well to cytotoxic therapy and plasmapheresis and achieved remission by 6 months. The findings suggest an overlap syndrome of IgA nephropathy-associated type 4 crescentic glomerulonephritis that resembles the former histologically and the latter in its potential to respond to aggressive therapy if detected relatively early in its course.
Keywords
Anti-GBM
ANCA
crescents
IgA nephropathy
rapidly progressive glomerulonephritis
Introduction
Rapidly progressive glomerulonephritis (RPGN) is defined as glomerular disease characterized by extensive crescent formation (usually over 50%) as the principal histologic finding and a rapid loss of renal function (usually a 50% decline in glomerular filtration rate [GFR] within 3 months) as the clinical correlate.[1] Pauci-immune vasculitis due to antineutrophil cytoplasmic antibodies (ANCA) with or without anti-glomerular basement membrane (anti-GBM) antibody is commonly associated with crescentic glomerulonephritis (GN).[1] They present with proteinuria, hematuria, and rapidly worsening renal failure with pulmonary alveolar hemorrhage. RPGN due to immune complex diseases like crescentic IgA nephropathy (IgAN), systemic lupus erythematosus, and infection-related GN (IRGN) are classified to be type 2.[1] Anti-GBM with ANCA is sometimes referred to as type 4 RPGN. There are rare reports of ANCA with IgAN[2] and anti-GBM with IgAN.[3] We herein report a rare case of double-positive type 4 RPGN along with significant IgA deposits presenting as nephrotic range proteinuria and crescentic GN.
Case Report
This 58-year-old hypertensive lady presented with edema, oliguria for a short duration. She denied a history of skin rashes, altered urine color, pain abdomen, arthralgia, hemoptysis, or fever. On examination, she had hypertension with anasarca. She had active urine sediment with nephrotic range proteinuria (4 g/day). Her other blood parameters including complete hemogram, liver function test, iron studies, and lactate dehydrogenase (LDH) were normal. She had significant hypoalbuminemia (2.6 gm/dL), elevated renal parameters (serum creatinine = 3.5 mg/dL, urea; 81 mg/dL). Her baseline renal function had been normal and unfortunately, we could not find a relevant urine examination report in her clinical records. Her ultrasonography showed normal-sized kidneys and she was subjected to renal biopsy. Her RPGN work up revealed perinuclear-antineutrophil cytoplasmic antibodies (p-ANCA) to be positive with an anti-myeloperoxidase titer of 50.45 RU/mL (<20/mL– normal) and positive anti-GBM antibody with titers of 55 RU/mL (<20 RU/mL). ANA by immunofluorescence method was negative with normal complement levels.
Her renal biopsy had seven glomeruli, none globally sclerotic, mesangial hypercellularity in all glomeruli, segmental sclerosis of capillary tuft in two glomeruli, cellular crescent in one glomerulus [Figures 1 and 2], no endocapillary proliferation or necrotizing lesion, fibrous intimal proliferation in the arteries, interstitial fibrosis and tubular atrophy (IFTA) of about 10% of the core. Immunofluorescence showed mesangial IgA (3+) and C3 (3+) deposition [Figure 3], M1E0S1T0 - C1 (MEST-C scoring)[4] with linear IgG (3+) deposition along the glomerular basement membrane [Figure 2] with no light chain restriction. A High-resolution computed tomography (CT) chest did not reveal any lung involvement. She received pulsed methylprednisolone, IV cyclophosphamide according to CYCLOPS protocol.[5] She was treated with five sessions of cascade double-filter plasma exchange (PEX). Her repeat anti-GBM titers, once after completion of PEX and second on follow-up after 3 months, were negative. Currently, she is on maintenance immunosuppression with oral prednisolone and azathioprine. Her kidney function has improved to a serum creatinine of 1.4 mg/dL at 6 months and she continues to be on close follow-up.
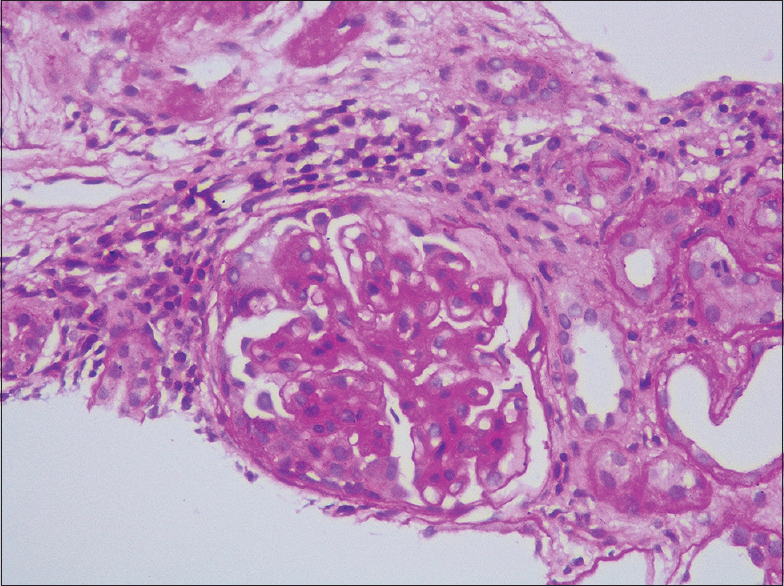
- Periodic Acid-Schiff stain showing one cellular crescent (400×)
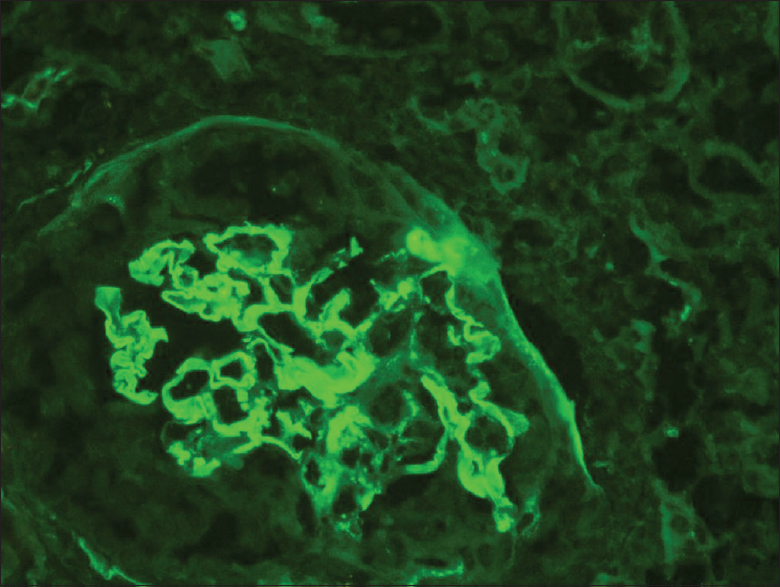
- Immunofluorescence stain showing linear deposition of IgG (3+) along glomerular basement membrane (400×)
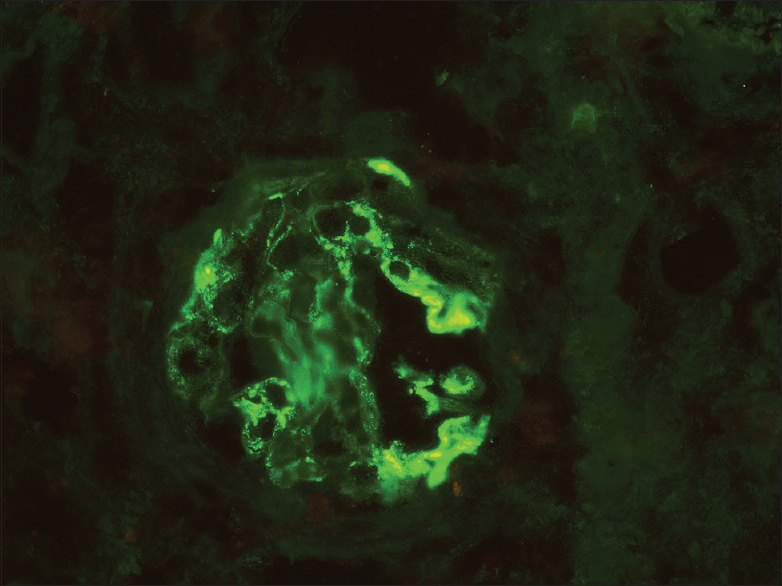
- Immunofluorescence stain showing mesangial IgA (3+) deposition (400×)
Discussion
RPGN is not an uncommon renal disease, accounting for around 8.1% of renal biopsies according to the largest registry available i.e., the Spanish registry which had around 21774 biopsied patients.[1] Golay et al. in a single-center Indian study showed that the occurrence of RPGN is around 26.15%.[6] Some historic reports are suggesting that anti-GBM antibodies would be present in 5–14% of patients with ANCA disease and ANCA antibodies in the range of 30–38% of patients with anti-GBM disease.[7] Ganesan showed an incidence of type 4 RPGN in India to be around 2.3% in 265 biopsy-proven RPGN patients.[8]
Anti-GBM disease classically presents with RPGN, while pulmonary hemorrhage occurs in 34–62% of patients.[9] The causal relationship of anti-GBM GN and IgAN till date remains unclear, with one hypothesis being that the IgA-related immune complex may encourage immunologic and inflammatory events which may result in conformational changes and exposure of the GBM antigens which could lead to the development of anti-GBM antibody.[3] Nonetheless, it is extremely difficult to prove whether anti-GBM disease developed secondary to IgAN or as an incidental complication because there does not exist a marker to distinguish primary from the secondary anti-GBM disease. In this regard, whether IgG4 predominance on biopsy relates to anti-GBM disease developed secondary to IgAN warrants future study. The pathophysiological condition of anti-GBM disease before a clinical presentation is unknown. In our case, multiple immunofluorescences labeling on the biopsy revealed linear IgG deposition along the glomerular capillary walls. In classic anti-GBM nephritis, C3 staining is seen in 78–96% cases and light microscopy shows >50% crescents[10] but our case neither had C3 staining along GBM nor >50% crescents which could suggest the presence of an atypical variant of anti-GBM. In a case series of 20 anti-GBM patients by Nasr. et al.[11] no case with >50% crescents was seen while one case of >20% crescent and mesangial hypercellularity analogous to our case was present. In the case of IgAN complicated by anti-GBM disease, Yamaguchi et al. conjectured that the pathological features of IgAN may not be observed since the number of glomeruli free from destruction may be very limited.[12] Therefore, the coexistence of IgAN and anti-GBM disease may be more common than what is being reported.
Lim et al. in his study found that among IgAN cases, approximately 15% were ANCA-positive with predominant p-ANCA pattern.[13] Bantis et al. reported the incidence of ANCA and IgAN is around 0.2–2%.[2] O'Donoghue found that 2% of patients with IgAN showed serum IgG-ANCA positivity and these patients had slowly progressive renal failure, without crescents or focal necrosis in their renal biopsies.[14] Very little is known about the prognosis of this “dual positivity” (ANCA serology and mesangial IgA deposits). Some of the available reports suggest a poor prognosis while others suggest a quite favorable prognosis.[13] The association between ANCA-associated crescentic GN and IgAN may be a purely fortuitous overlap of the two lesions that are among the more commonly observed glomerular diseases on renal biopsy.[15] The inflammatory response to IgA-containing immune complexes may predispose some patients to develop ANCA and this may be the pathogenesis.
The concurrence of mesangial IgA deposits with p-ANCA and anti-GBM is uncommon. The possibility of a higher incidence of asymptomatic IgAN in the population may explain this unique occurrence. In a large follow-up study by Das et al.,[16] IgAN though uncommon has shown to have increased incidence in two decade-long data from 1990 to 2008 in renal biopsy reports. Whether this epidemiological increase is a part of better and early biopsy habits of the treating nephrologists is yet to be ascertained. Even in the above study, the rate of double positivity was not mentioned but crescentic GN was only 0.4%.[16]
After performing an extensive literature search on the occurrence of triple-positive RPGN, a solitary case report by Divyaveer et al. was found[17] wherein a first-trimester pregnant lady presented with dialysis requiring RPGN, underwent medical termination of pregnancy and remained dialysis-dependent even after receiving plasmapheresis and induction therapy according to CYCLOPS protocol. In our patient, we achieved remission which may be due to the lack of chronicity and presence of cellular crescents in our biopsy as compared to the above lady who had severe IFTA along with fibrocellular crescents. In our case, the lady presented as RPGN with nephrotic range proteinuria and controlled hypertension. A combination of serological testing and renal biopsy revealed the unique triple positivity. Isolated ANCA positivity has been seen in around 40% of patients of anti-GBM disease[18] and may not present with typical pulmonary-renal syndrome hence considered to be a vasculitic variant of the anti-GBM disease.[19] It was shown in a rat model that Myeloperoxidase autoantibodies may aggravate subclinical anti-GBM disease transforming mild glomerular disease into a severe form.[20] Although our biopsy did not reveal features of vasculitis it still cannot be completely ruled out due to the following reasons; the age of the patient, clinical presentation, response to treatment, very high p-ANCA titers, under-represented kidney biopsy (ANCA being a focal disease needs minimum 10 glomeruli), and lack of systemic vessel biopsy sample. Our patient presented as an atypical variant of anti-GBM phenotype but responded reasonably well to immunosuppressive therapy as an ANCA vasculitis patient. We intend to follow-up with our patient for IgAN.
Conclusions
Triple positivity RPGN is a rare phenomenon. The exact incidence, pathogenesis, management, and prognosis are not clear. The pathogenesis may be an interrelated occurrence that may be induced by infection or be a chance finding hence needs further evaluation. Immunosuppressive therapy may prove beneficial for these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Spanish Registry of Glomerulonephritis. Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004;66:898-904.
- [Google Scholar]
- Is presence of ANCA in crescentic IgA nephropathy a coincidence or novel clinical entity? A case series. Am J Kidney Dis. 2010;55:259-68.
- [Google Scholar]
- Recurrence of antiGBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol. 1998;49:124-8.
- [Google Scholar]
- The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546-56.
- [Google Scholar]
- Pulse versus daily oral CYCLOPHOSPHAMIDE for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med. 2009;150:670-80.
- [Google Scholar]
- The spectrum of glomerular disease in single centre: A clinicopathological correlation. Indian J Nephrol. 2013;23:168-75.
- [Google Scholar]
- Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37:965-70.
- [Google Scholar]
- A study of clinicopathological features and outcomes of crescentic glomerulonephritis. Int J Res Med Sci. 2020;8:915-20.
- [Google Scholar]
- Anti-glomerular basement membrane disease: Outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine (Baltimore). 2011;90:303-11.
- [Google Scholar]
- Anti-glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am J Clin Pathol. 2006;125:445-50.
- [Google Scholar]
- The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 2016;89:897-908.
- [Google Scholar]
- A case report of the anti-glomerular basement membrane glomerulonephritis with mesangial IgA deposition. CEN Case Rep. 2013;2:6-10.
- [Google Scholar]
- Diagnostic usefulness of anti-neutrophil cytoplasmic autoantibody serology. Comparative evaluation of commercial indirect fluorescent antibody kits and enzyme immunoassay kits. Am J Clin Pathol. 1999;111:363-9.
- [Google Scholar]
- Anti-neutrophil cytoplasmic antibodies in IgA nephropathy and Henoch- Schonlein purpura. Nephrol Dial Transplant. 1992;7:534-8.
- [Google Scholar]
- A re-evaluation of routine electron microscopy in the examination of native renal biopsies. J Am Soc Nephrol. 1997;8:70-6.
- [Google Scholar]
- Pattern of biopsy-proven renal disease in a single center of south India: 19 years' experience. Indian J Nephrol. 2011;21:250-7.
- [Google Scholar]
- An unusual case of rapidly progressing glomerulonephritis in pregnancy;” triple positivity” or a co incidence? J Nephropathol. 2017;6:272-4.
- [Google Scholar]
- Incidence and features of dual anti-GBM-positive and ANCA-positive patients. Nephrology. 2011;16:725-9.
- [Google Scholar]
- Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253-62.
- [Google Scholar]
- Autoantibodies to myeloperoxidase aggravate mild anti-glomerular-basement-membrane-mediated glomerular injury in the rat. Am J Pathol. 1996;149:1695-706.
- [Google Scholar]







