Translate this page into:
A Study of Association of Urinary Nephrin with Albuminuria in Patients with Diabetic Nephropathy
Address for correspondence: Dr. Santhi Silambanan, SRMC and RI, Sri Ramachandra Institute of Higher Education Research (Deemed to be University), Porur, Chennai - 600 116, Tamil Nadu, India. E-mail: santhisilambanan@sriramachandra.edu.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Aims:
Diabetes mellitus and its complications are associated with high mortality and morbidity. Early detection is mandatory to improve quality of life years in patients with diabetic nephropathy. Hyperglycaemia disrupts podocytes, both structurally and functionally, leading to excretion of nephrin which is present in the glomerular filtration barrier. This study was undertaken to find out whether urinary nephrin is a better indicator of podocyte injury than albuminuria in patients with diabetic nephropathy.
Methods:
The study included 125 type 2 diabetes mellitus patients as cases categorized into three groups, depending upon albumin excretion. Age and sex matched 45 individuals without diabetes mellitus were chosen as the control group. The study protocol was approved by Institutional Ethics committee. Microalbumin was estimated by immunoturbidometry and urinary nephrin by ELISA. ANOVA and Tukey post-hoc tests were done to compare the data between the groups. Correlation studies were done. Odds ratio for nephrin was calculated. P value less than 0.05 was considered statistically significant. The statistical analyses were performed with SPSS software version 13.0.
Results:
The urinary nephrin was found to be proportionately increased from normoalbuminuria to macroalbuminuria and it was statistically significant, with sensitivity of 92.5% and specificity of 76.7%, the cut-off value of urinary nephrin was 97.5ng/mL.
Conclusion:
Albuminuria has been used as an independent predictor of diabetic nephropathy. The statistical significant difference between the groups inferred that urinary nephrin excretion increased even in the stage of normoalbuminuria. Nephrin expression and its phosphorylation get altered by hyperglycaemia, contributing to renal damage. Nephrin was found to be a sensitive marker of early kidney dysfunction in diabetic patients.
Keywords
Albuminuria
diabetic nephropathy
GFR
glomerular filtration barrier
glomerular injury
nephrin
podocyte injury
Introduction
Diabetes mellitus (DM) is a major metabolic disorder amounting to 35% of the deaths across the world.[1] International Diabetes Federation (IDF) has reported that number of diabetics in India is expected to increase from 41 million in 2006 to 70 million in 2025.[23] In Southeast Asia around 80 million were affected by type 2 diabetes mellitus (T2DM) in 2017 which might increase to 151 million in 2045. In the recent times, T2DM is found to be prevalent among younger age and non-obese individuals also and the course of disease progression is also quicker with adverse outcomes.[1] Studies done in UK had shown that immigrants from Pakistan, Bangladesh and India were at a higher risk of developing DM.[4]
Early deterioration in kidney function is assessed by measuring albumin excretion in urine. Long standing diabetes leads to incipient albuminuria or microalbuminuria (30-300 mg/g of creatinine) which later leads to overt proteinuria or macroalbuminuria (>300mg/g of creatinine). In the later stages, kidney function is assessed by estimating GFR. eGFR has been calculated from serum creatinine and other variables such as age, sex, race, and body size.[5]
Glomerular dysfunction is a fundamental feature of kidney disease [Figure 1].[6] Slit diaphragm (SD) is composed of nephrin, neph 1 and podocin. Nephrin maintains the glomerular slit diaphragm. It is also involved in signaling of processes which maintains podocyte polarity, cytoskeletal organization, calcium mechano-sensing and SD turnover. To carry out various signalling mechanisms nephrin undergoes phosphorylation in its various tyrosine residues [Figure 2].[6] In T2DM, the foot processes of injured podocytes retract into broader effacement, with loss of SD and nephrin is downregulated or mislocalized.[7] The acto-myosin network of podocytes relocates to the basolateral surface of the cell, and SDs are replaced by tight junctions.[68] These changes lead to thickening of glomerular and tubular basal membranes, accumulation of collagen with resultant mesangial expansion, interstitial morphological changes and hyalinization of glomerular arterioles.[9] This study was conducted to find out the association of nephrin with albuminuria and also whether nephrin is a better predictor of diabetic nephropathy than albuminuria in type 2 diabetics mellitus.
![Glomerular filtration barrier[6]](/content/170/2021/31/2/img/IJN-31-142-g001.png)
- Glomerular filtration barrier[6]
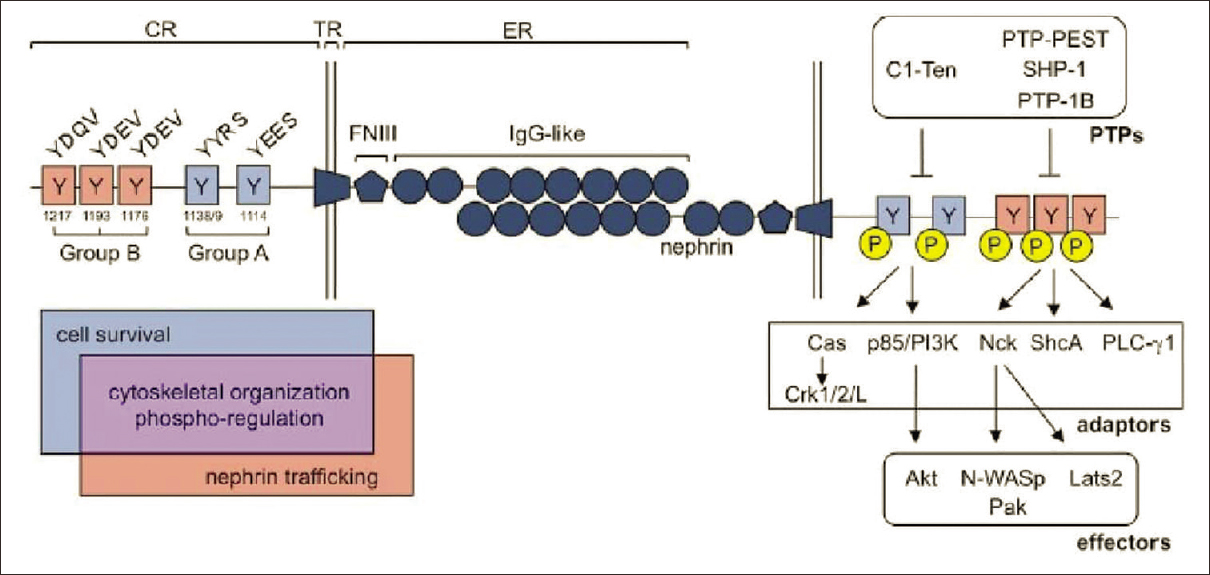
- Nephrin tyrosine phosphorylation regulates a diverse group of signaling processes within the podocyte6
Methods
This case control study was conducted in the Department of Biochemistry of Sri Ramachandra Institute of Higher Education and Research. The study participants were in the age group of 45 to 60 years of both sexes. Apparently healthy, age and sex matched individuals (n = 45) without diabetes mellitus were chosen as the control group (Group I). Individuals with type 2 diabetes mellitus of more than 5 years duration (n = 125) were chosen as the cases. Based on urinary albumin excretion, the cases were categorized into three groups: Normoalbuminuria (Group II, n = 45), Microalbuminuria (Group III, n = 40) and Macroalbuminuria (Group IV, n = 40). The study participants were included from the Department of General Medicine at G.S.L. Medical College and Hospital, Rajahmundry. The patients were not been subjected to renal biopsy to study the pathology of nephropathy. Pregnant women, individuals with hypertension, alcoholics, smokers, and individuals on ACE or ARB drugs, were excluded from the study. The study protocol was approved by the Institutional Ethics committee of both Sri Ramachandra Institute of Higher Education and Research, Chennai and G.S.L. Medical College and Hospital, Rajahmundry, in accordance with the declaration of Helsinki. After getting written informed consent from all study participants, they were included in the study.
Blood samples were taken for estimating glucose, urea, creatinine, and eGFR. Spot urine samples were obtained for estimation of nephrin, microalbumin and albumin/creatinine ratio. Glucose, urea and creatinine were estimated by standard methods. Micro albumin was estimated by immunoturbidometric method, urinary nephrin by ELISA (Cat.NoE109Hu) and eGFR was calculated by MDRD equation: 1.86x (creatinine/88.4)-1.154 x (Age)-0.203 x (0.742 if female). Since the data were found to follow normal distribution by Kolmogorov Smirnov test, the data were expressed as mean and SD. Comparison of data was done by ANOVA. Tukey post-hoc test was done to analyze statistical significant difference between the groups. Correlation studies were done between nephrin and other biochemical parameters. Odds ratio was calculated for urinary nephrin. P value less than 0.05 was considered statistically significant. ROC curve analysis was done to arrive at the cutoff level of urine nephrin in patients with diabetic nephropathy. The statistical analyses were performed with SPSS software version 13.0.
Results
The biochemical parameters are shown in Table 1. All the study participants were found in the age group of 45 to 60 years. Then mean duration of diabetes in groups II to IV is 8 years. In the present study, fasting and postprandial glucose values were in the normal range in the control group (group I), but markedly elevated in all the case groups with P value <0.001 [Table 1]. Tukey post-hoc test shows significant difference in both fasting and postprandial glucose except between microalbuminuria and macroalbuminuria groups (groups II and III) [Table 2].
| Controls (Group I) n=45 | Normoalbuminuria (Group II) n=45 | Microalbuminuria (GroupIII) n=40 | Macroalbuminuria (Group IV) n=40 | ANOVA P | |
|---|---|---|---|---|---|
| Age (years) | 49.57±6.08 | 50.9±5.59 | 52.82±6.64 | 53.7±6.00 | 0.009 |
| DM duration (years) | 0 | 8.13±2.17 | 8.85±2.17 | 8.80±1.68 | <0.001** |
| Fasting plasma glucose (mg/dL) | 75.71±9.03 | 133.60±31.80 | 123.88±21.88 | 157.28±21.01 | <0.001** |
| Postprandial plasma glucose (mg/dL) | 113.64±13.45 | 195.87±46 | 181.38±30.8 | 222.13±37.48 | <0.001** |
| Microalbumin (mg/g) | 25.07±3.27 | 24.88±3.23 | 84.70±35.7 | 352±41.91 | <0.001** |
| Serum Creatinine (mg/dL) | 0.78±0.11 | 0.918±0.14 | 1.04±0.14 | 2.18±0.34 | <0.001** |
| UrineCreatinine (g/L) | 0.97±0.19 | 1.02±0.18 | 0.93±0.22 | 0.84±0.07 | <0.001** |
| ACR (mg/g) | 25.08±4.06 | 25.46±3.25 | 84.90±43 | 351±35.1 | <0.001** |
| Serum Urea (mg/dL) | 37.51±4.42 | 33.44±6.89 | 28.03±7.47 | 45.33±10.09 | <0.001** |
| eGFR (ml/mt) | 107.56±14.73 | 86.62±20.83 | 75.60±12.69 | 31.33±7.12 | <0.001** |
| Urine Nephrin (ng/mL) | 66.18±8.29 | 97.55±10.90 | 116±19.17 | 237.55±74.99 | <0.001** |
The biochemical parameters were expressed as Mean±SD; P<0.5 was considered statistically significant; **Highly significant
| Group I Vs II (P) | Group I Vs III (P) | Group I Vs IV (P) | Group II Vs III (P) | Group II Vs IV (P) | Group III Vs IV (P) | |
|---|---|---|---|---|---|---|
| Age (years) | 0.705 | 0.071 | 0.011 | 0.492 | 0.165 | 0.918 |
| DM duration (years) | <0.001** | <0.001** | <0.001** | 0.376 | 0.441 | 1.000 |
| Fasting plasma glucose (mg/dL) | <0.001** | <0.001** | <0.001** | 0.196 | <0.001** | <0.001** |
| Postprandial plasma glucose (mg/dL) | <0.001** | <0.001** | <0.001** | 0.209 | 0.003** | <0.001** |
| Microalbumin (mg/g) | 1.000 | <0.001** | <0.001** | <0.001** | <0.001** | <0.001** |
| Serum Creatinine (mg/dL) | 0.012* | <0.001** | <0.001** | 0.021* | <0.001** | <0.001** |
| Urine Creatinine (g/L) | 0.497 | 0.732 | 0.005** | 0.083 | <0.001** | 0.108 |
| ACR (mg/g) | 1.000 | <0.001** | <0.001** | <0.001** | <0.001** | <0.001** |
| Serum Urea (mg/dL) | 0.049* | <0.001** | <0.001** | 0.005** | <0.001** | <0.001** |
| eGFR (ml/mt) | <0.001** | <0.001** | <0.001** | 0.005** | <0.001** | <0.001** |
| Urine Nephrin (ng/mL) | 0.001** | <0.001** | <0.001** | 0.120 | <0.001** | <0.001** |
**Highly significant; *Significant
Urinary albumin creatinine ratio was found to be 25.07 ± 3.27mg/g and 24.88 ± 3.23 mg/g in Groups I and II respectively and they were within the normal limits with no significant difference between the groups. In groups III and IV, urinary albumin creatinine ratio was 84.70 ± 35.7mg/g and 352 ± 41.91mg/g of respectively [Table 1]. There was a statistical significance in urine albuminuria between the groups as shown by the P value of <0.001. Table 2 shows that there was significant difference between the groups except between groups I and II, showing that rate of excretion of albumin increases after the disease has progressed to a certain extent. Serum creatinine was elevated in group IV and within reference interval in the other three groups. But there was gradual increase in serum creatinine across the groups as shown by the P value of <0.001. Urinary nephrin was proportionately increased from group I to group IV [Table 1]. There was significant difference between the groups with P value of <0.001 [Table 2].
Odds ratio was calculated to find out the significance of nephrinuria in patients with and without significant albuminuria. Data from controls (group I) and normoalbumineuria (group II) were pooled together as the control group (n = 90) since all the participants had normoalbuminuria. And data from group III (microalbuminuria) and group IV (macroalbuminuria) were grouped as the case group (n = 80) since all the patients had significant albuminuria. Odds ratio was found to be 32.92 with 95% confidence interval (13.79 – 93.58) and P < 0.0001.
Figure 3 shows the correlation of urinary nephrin with other biochemical markers. Receiver Operating Characteristics curves were done. For this statistical analysis, groups I & II were combined as normoalbuminuria (n = 90) and groups III & IV with significant albuminuria (n = 80) were pooled together as a single group of increased albuminuria [Figure 4].
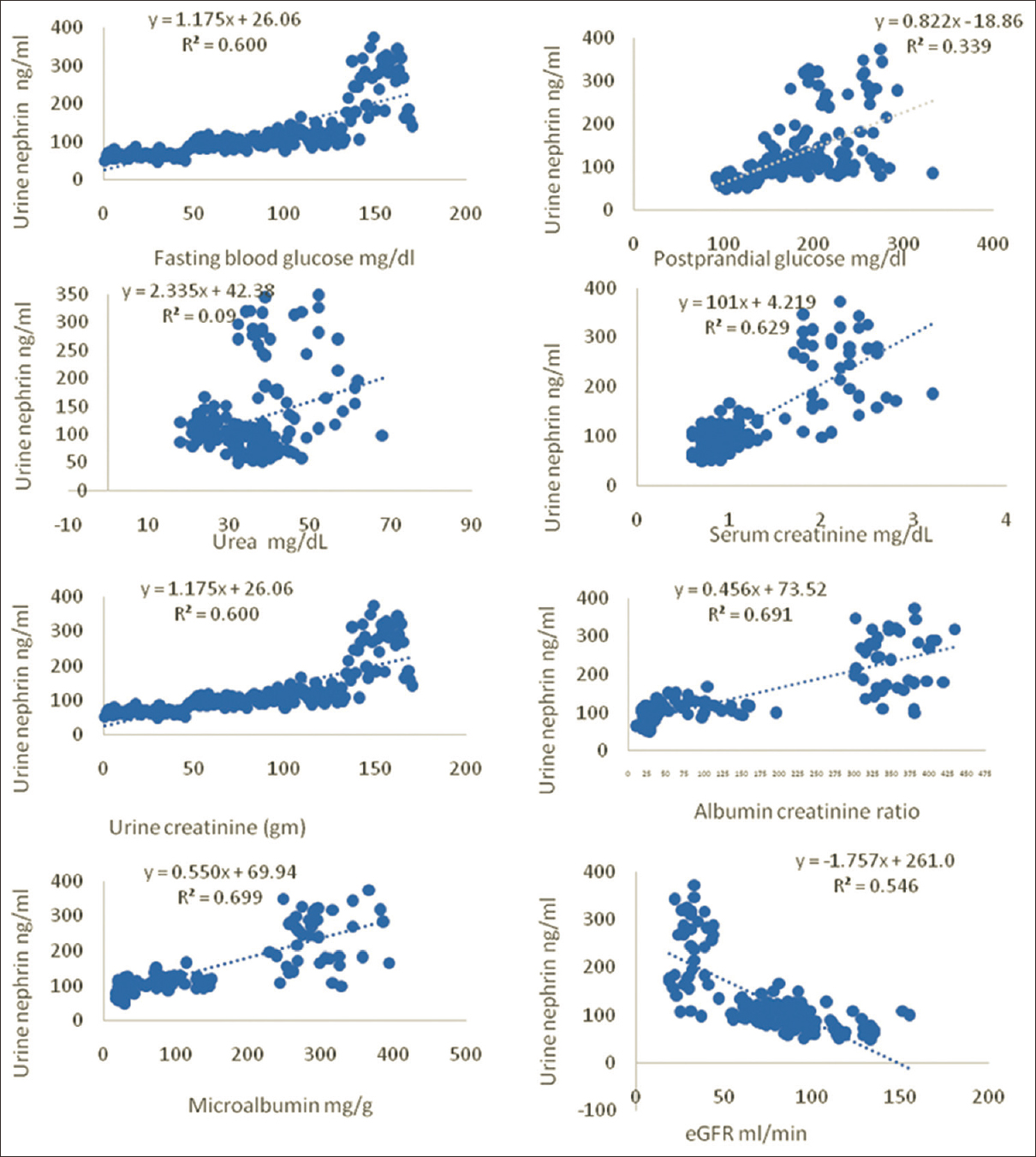
- Correlation graphs showing the relationship of urine nephrin with other biochemical markers
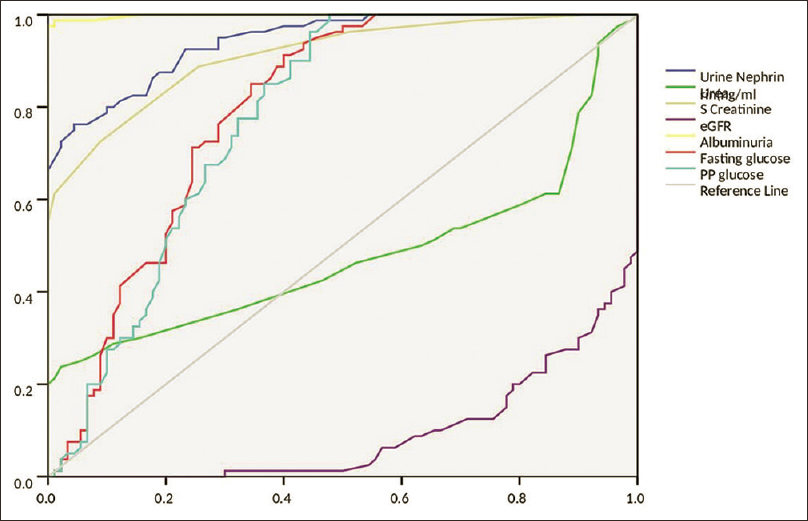
- ROC analysis of the biochemical parameters
Discussion
The global prevalence of type 2 diabetes mellitus is on the rise. A recent Indian study shows that 46.5% of the population with diabetes mellitus are undiagnosed.[4] As diabetes is asymptomatic, patients are diagnosed only when they develop the macrovascular and microvascular complications. Hence, during the diagnosis of diabetes, screening for its complications at the time of diagnosis of DM is mandatory. This helps in slowing down the progression of the disease by aggressive control of hyperglycemia, hypertension, dyslipidemia, and use of drugs that control albuminuria.[10] Hyperglycemia in diabetes mellitus leads to increased adiposity and inflammation, which predisposes to podocyte insulin resistance and glomerular dysfunction.[2]
Fasting plasma glucose levels were above the diabetic cutoff levels in all the case groups II, III and IV. Whereas postprandial glucose level was above the diabetic cutoff level only in Group IV but not in groups II and III, probably because of better control of the diabetic status in groups II and III [Table 1]. Table 2 shows that there was significant difference between the groups except between groups II and III, who have almost the same fasting and postprandial glucose levels. This could be due to diabetic status remaining unaltered from normoalbumineuria to microalbuminuria but increased in group IV, where the patients had macroalbuminuria. Thus, uncontrolled diabetes status in group IV has resulted in progressive renal damage with macroalbuminuria.
Urinary albumin was found to be within normal limits in groups I and II; whereas in groups III and IV, they were in the microalbuminuria and macroalbuminuria range respectively. There were significant differences in albuminuria between the groups as shown by the P value of <0.001 [Table 1]. Table 2 shows there was significant difference in urinary albumin between the groups. There was no significant difference between groups I and II because both groups of individuals had normal albuminuria. Albuminuria is a continuum across the progression of the disease.[1112] It is universally considered to be the indicator of onset of DN.[2] Albuminuria is associated with endothelial dysfunction. In 20-40% of T2DM, microalbuminuria starts within 10–15 years of onset of DM, which in another 5 years would result in macroalbuminuria.[1113] With the control of the risk factors, patients with microalbuminuria have more chances of spontaneously reverting back to normal. Complete remission is highly unlikely in patients with macroalbuminuria, but the extent of progression of DN can be delayed.[14]
Albumin which escapes from the glomerular filtrate is reabsorbed by the cubilin–megalin protein complex in the proximal tubular cells. Reabsorbed albumin causes further renal damage through activation of oxidative stress and inflammation ultimately leading to tubular cell apoptosis, monocyte infiltration, complement activation, accumulation of extracellular matrix in the interstitium resulting in renal fibrosis and renal function loss.[151617] Due to biological variability in urinary albumin excretion, two of three specimens collected within a 3- to 6-month period should be abnormal before arriving at a conclusion of increased albuminuria.[131819] Albuminuria levels are variable in circumstances like collection of urine on different days, exercise, hyperglycemia, urinary tract infections, inflammation, water consumption, menstruation, obesity, exercise, diet, smoking and posture.[20] There are few limitations which discourages albuminuria from being a potential marker of DN such as lack of standardized methodology for estimation, lack of uniformity in reporting, lack of age and sex specific reference intervals.[2021]
In the present study, serum creatinine was elevated only in group IV, but there was progressive increase as shown by the P value of <0.001 [Table 1]. Serum creatinine remains unaltered until 50-60% of the kidney damage has taken place.[1517] In this study, albumin creatinine ratio (ACR) was within normal in Groups I and II, but increased in groups III and IV. There were statistical significant differences between the groups except between groups I and II. Urea was within normal limits in groups I to III, whereas in group IV, there was borderline elevation which was proportionate with elevated serum creatinine. Urea itself induces molecular changes relating to insulin resistance, free radical production, apoptosis and disruption of the protective intestinal barrier.[22] In this study, there was progressive decline in eGFR from groups II to IV. In group II, it was in the borderline of normal (86.62 ± 20.83 ml/mt). In the macroalbuminuria stage of group IV, there is drastic decrease in eGFR (31.33 ± 7.12 ml/mt). Thus, it was inferred that urea and eGFR are not biomarkers of early stages of DN but can be used in conjunction with albuminuria.
Urine nephrin was found to be 66.18 ± 8.29 ng/mL. 97.55 ± 10.90 ng/mL, 116 ± 19.17 ng/mL and 237.55 ± 74.99 ng/mL in groups I to IV respectively. There was statistically significant difference with a P value of <0.001 [Table 1]. There was significant difference between groups I and II showing that urine nephrin started increasing even in the stage of normoalbuminuria [Table 2]. This showed that nephrin is a better biomarker of early diabetic nephropathy in T2DM patients; probably earlier than urinary albumin. Phosphorylation of tyrosine residues of nephrin mediates recovery of injured glomerulus and thus preserves glomerular function.[23] Decrease in nephrin expression as found in diabetes, impairs the ability of podocytes to recover following injury making them susceptible to detachment.[24] Nephrin is a better indicator of early kidney injury in DN even when there is normoalbumineuria. In T2DM, around 35-57% of patients with chronic kidney disease (CKD) do not present with albuminuria. It has been found that nonalbuminuric CKD has been associated with advanced glomerular lesions compared with patients with albuminuric CKD. This type of presentation is common in females, old age, and in the presence of associated hypertension, neuropathy and cardiovascular disease.[25]
Podocyte injury is found in minimal change disease, membranous and collapsing glomerulopathy, crescentic glomerulonephritis, focal segmental glomerulosclerosis, diabetic nephropathy, and lupus nephritis. In the early stages of podocyte injury, there is detachment of podocytes from glomerular basement membrane. If the causative factor persists, it can result in severe and progressive glomerular injuries. Hence, early recognition of podocyte injury is mandatory to preserve glomerular function. In both human and animal models, there was significant nephrinuria which was earlier than microalbuminuria. Nephrinuria might have a role in causing proteinuria. Nephrin excretion was found to be significant in diabetic patients with normoalbuminuria whereas the control group who were non-diabetics, were not excreting nephrin in urine. They found nephrinuria is a marker of preclinical diabetic nephropathy.[26] The risk of serious renal damage in patients with essential hypertension is low when compared to cardiovascular complications. In patients with diabetes mellitus, whatever measures are taken to retard renal disease progression is futile despite the blood pressure being normal.[27] Hypertension per se leads to glomerular injury only in the later stages compared to diabetes mellitus. In diabetes mellitus when there is associated hypertension and obesity glomerular hyperfiltration sets in. Glomerular hyperfiltration is a well characterized to be an early feature of diabetes. This is because the pathogenesis leading to hyperfiltration in diabetes is different from that of hypertension. In diabetes mellitus there is increased reabsorption of glucose in the proximal convoluted tubule, whereas in hypertension it is sodium mediated glomerular injury.[28]
Nephrin showed positive correlation with albuminuria, albumin creatinine ratio, serum creatinine and fasting plasma glucose and negative correlation with eGFR [Figure 3]. Nephrinuria is a better indicator of renal insufficiency in normoalbuminuric T2DM. Increased glomerular hydrostatic pressure in early diabetes mellitus contributes to nephrinuria.[29]
Odds ratio was calculated to find out the significance of nephrinuria in patients with and without significant albuminuria. Odds ratio was found to be 32.92, with 95% confidence interval (13.79-93.58) and P < 0.0001. Urinary nephrin was an early indicator of podocyte injury even when the urinary albumin excretion was not significantly elevated.
ROC curve [Figure 4] showed the cutoff level of urine nephrin was 97.5 ng/ml with the sensitivity of 92.5% and specificity of 76.7%; the cutoff level of microalbuminuria was 31 mg/g of creatinine with the sensitivity of 97.5% and specificity of100% and the cutoff level of serum creatinine was 0.95 mg/dL with the sensitivity of 88.8% and specificity of 74.4%. Area under the Curve (AUC) for urine nephrin, albuminuria and serum creatinine were found to be 0.943, 0.998 and 0.912 respectively. Even though AUC for urine albuminuria was the highest of 0.998, it could not satisfy the criteria of early diagnosis of DN since it is not elevated in normoalbuminuria. The threshold of albuminuria varies greatly between individuals; DN can progress without significant albuminuria.[14] Nephrin expression and nephrin tyrosine phosphorylation are significantly reduced by diabetes earlier than the onset of microalbuminuria. Further research on podocyte metabolism might confirm nephrinuria to be a biomarker of pre-clinical DN.[30]
Based on the cutoff value of 97.5ng/ml for nephrin, 51.1% of diabetic individuals with normoalbumineuria had increased nephrin (Group II), 85% of diabetic individuals with microalbuminuria had increased nephrin (group III) and 100% of diabetic individuals with macroalbuminuria had increased nephrin (Group IV). This showed that 51.1% diabetic individuals despite having normal albumin excretion showed increased nephrinuria. Urine nephrin is an early marker of podocyte injury in patients with diabetes mellitus.
According to Yuping Wang, nephrin in normal pregnancy was found to be 86 ± 22 ng/ml which is almost the as per the present of 66.18 ± 8.29 ng/mL in the control group.[31] According to Irena Kostovska nephrin levels were found to be 160.5 ± 58ng/mL, 444.6 ± 237.6 ng/mL, 983.3 ± 1182.5 ng/mL and 1086.5 ± 550 ng/mL in controls, normoalbuminuria, microalbuminuria and macroalbuminuria respectively. The levels were higher compared the nephrin levels obtained in this study probably due to variations in ethnicity, diet, environmental and methodology used. But the proportionate variation in urine nephrin levels were found in the present is similar to the study of Irena Kostovska. Also, the number of study participants in groups III and IV are fewer compared to the present study.[32] Since multiple pathophysiological processes are involved in diabetic kidney disease, it may be difficult for a single biomarker to predict the disease. It is better to develop a panel of biomarkers that capture several pathophysiological processes which might improve prediction of DN progression.[3334]
Conclusion
Early identification of the complications of DM will aid in the management of complications in addition to controlling hyperglycemia. Diabetes per se is not fatal. High mortality and morbidity are due to its complications such as cardiovascular disease and chronic kidney disease. In patients with T2DM, even in later stages of DN albuminuria remains within the normal range which is highly misleading. Urinary nephrin with high sensitivity and specificity is found to be promising marker for early diagnosis of diabetic nephropathy even before the stage of incipient albuminuria.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diabetic nephropathy: Perspective on novel molecular mechanisms. Trends EndocrinolMetab. 2016;27:820-30.
- [Google Scholar]
- Comparative study of the prevalence of type-2 diabetes mellitus in various demographic regions of Andhra Pradesh, India: A population based study. Int J MCH AIDS. 2016;5:103-11.
- [Google Scholar]
- Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectionalstudy. Lancet Diabetes Endocrinol. 2017;5:585-96.
- [Google Scholar]
- Estimationof glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of diet in renal disease study equation.? Diabetes Care. 2005;28:838-43.
- [Google Scholar]
- Nephrin signaling in the podocyte: An updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol. 2018;9:1-12.
- [Google Scholar]
- PACSIN2 accelerates nephrin trafficking and is up-regulated in diabetic kidney disease. FASEB J. 2017;31:3978-90.
- [Google Scholar]
- Podocyte lossand progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342-8.
- [Google Scholar]
- Research progress in signaling pathway in Diabetic Nephropathy. Diabetes Metab Res Rev. 2015;31:221-33.
- [Google Scholar]
- Cardiovascular risk factors, micro and macrovascular complications at diagnosis in patients with young onset type 2 diabetes inIndia: CINDI 2. Indian J Endocrinol Metab. 2016;20:114-8.
- [Google Scholar]
- Update on blood pressure control and renaloutcomes in diabetes mellitus. Curr Diab Rep. 2015;15:44.
- [Google Scholar]
- Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382-91.
- [Google Scholar]
- Control of albuminuria in overt diabetic nephropathy: Durability counts. Nephrol Dial Transplant. 2016;31:1371-3.
- [Google Scholar]
- Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76:516-23.
- [Google Scholar]
- Non-proteinuric rather than proteinuric renal diseases are theleading cause of end-stage kidney disease. Nephrol Dial Transplant. 2017;32:ii194-9.
- [Google Scholar]
- How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974-84.
- [Google Scholar]
- Useof protein: Creatinineratio in a random spot urine sample for predictingsignificant proteinuria in diabetes mellitus. Nepal Med Coll J. 2010;12:100-5.
- [Google Scholar]
- Progressive diabetic nephropathy. How useful is microalbuminuria?:Contra. Kidney Int. 2014;86:50-7.
- [Google Scholar]
- Chronic kidney disease and measurement of albuminuria or proteinuria: A position statement. Med J Aust. 2012;197:224-5.
- [Google Scholar]
- Urea and chronic kidney disease: The comeback of the century.(in uraemia research)? Nephrol Dial Transplant. 2018;33:4-12.
- [Google Scholar]
- Regulation of nephrin phosphorylationin diabetes and chronic kidney injury. Adv Exp Med Biol. 2017;966:149-61.
- [Google Scholar]
- Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS One. 2018;13:e0198013.
- [Google Scholar]
- The presence and consequence of non-albuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2015;38:2128-33.
- [Google Scholar]
- Pathophysiology of hypertensive renal damage-implications for therapy. Hypertension. 2004;44:595-601.
- [Google Scholar]
- Diabetic kidney disease-challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032-45.
- [Google Scholar]
- Nephrinuria associates with multiple renal traits in type 2 diabetes. Nephrol Dial Transplant. 2010;26:2508-14.
- [Google Scholar]
- Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: A cross sectional study. PLoS One. 2012;7:e36041.
- [Google Scholar]
- Increased urinary excretion of nephrin, podocalyxin, and βig-h3 in women with preeclampsia. Am J Physiol Renal Physiol. 2012;302:F1084-9.
- [Google Scholar]
- Urinary Nephrin is earlier, more sensitive and specific marker of Diabetic Nephropathy than Microalbuminuria. J Med Biochem. 2019;38:1-8.
- [Google Scholar]
- Biomarkers of renal disease and progression in patients with diabetes. J Clin Med. 2015;4:1010-24.
- [Google Scholar]
- Willthe future lie in multitude? A critical appraisal of biomarker panel studies onprediction of diabetic kidney disease progression. Nephrol Dial Transplant. 2015;30(Suppl 4):iv96-104.
- [Google Scholar]







