Translate this page into:
Acute interstitial nephritis due to proton pump inhibitors
Address for correspondence: Dr. Krishnaswamy Sampathkumar, Meenakshi Mission Hospital and Research Centre, Madurai - 625 107, India. E-mail: drksampath@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Proton pump inhibitors (PPI) are commonly prescribed for dyspepsia and acid peptic disease. Acute interstitial nephritis (AIN) is an uncommon though important side-effect of these classes of drugs. We describe four cases: three females and one male. PPIs implicated were pantoprazole in two, omeprazole and esomeprazole in one each. AIN developed after an average period of 4 weeks of drug therapy. The symptoms were vomiting, loin pain, and oliguria. Minimal proteinuria with pyuria were seen and the mean serum creatinine was 4.95 ± 4 mg/dl. Two patients required hemodialysis. Renal biopsy showed interstitial mononuclear, plasma cell and eosinophilic infiltrates in all cases. PPI was stopped and steroids were started in all. Renal recovery was total in two and partial in two. A high index of suspicion is required to diagnose PPI induced AIN. Renal biopsy for confirmation followed up by prompt steroid therapy results in renal functional improvement.
Keywords
Acute interstitial nephritis
corticosteroids
proton pump inhibitors
renal biopsy
Introduction
Proton pump inhibitors (PPI) which are benzimidazole derivatives have made a significant impact on the treatment of acid peptic disease. Omeprazole was the first PPI to be introduced for clinic use in 1989. One of the most commonly prescribed drugs the world over, they are preceived as safe with low side-effect profile. Acute interstitial nephritis (AIN) is being increasingly recognized as a complication of PPIs. These drugs are widely prescribed and sold over the counter for specific as well as empiric indications in India. Physicians consider them as renally safe agents, but this view needs to be reconsidered in the light of recent evidences. We describe here the first case series of renal toxicity of PPI from India.
Case Reports
Case 1
Our first index case was a 49-year-old female who was on follow-up for hypothyroidism, bronchial asthma, and acid peptic disease. She was taking omeprazole, eltroxin, deriphyllin for 8 weeks before being admitted with complaints of dysphagia and heart burn for 4 days. Here was no fever, loin pain or skin rash. Blood pressure was 140/80 mmHg. On admission, dipstick urinalysis showed 1+ protein and with 4-6 WBCs/hpf. Blood urea was 45 mg/dl and serum creatinine 1.6 mg/dl. Oliguria developed after 48 h and renal failure worsened. Renal ultrasonogram showed enlarged kidneys with increased cortical echoes. In renal biopsy the glomeruli were normal, but interstitium showed diffuse and dense lymphocytic infiltrates covering >90% of the core [Figure 1].
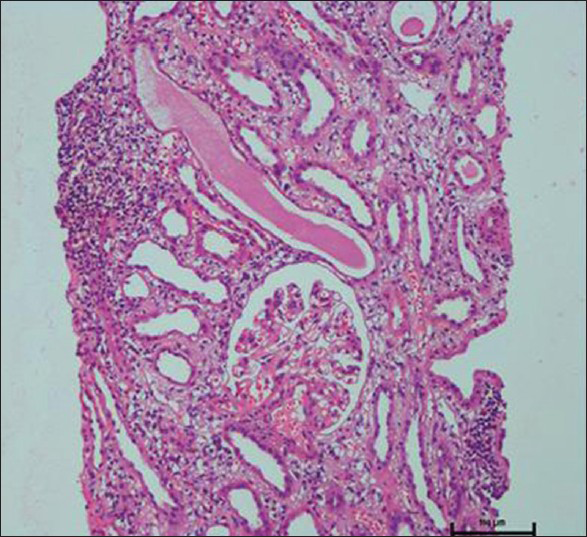
- Renal biopsy showing dense interstitial mononuclear infiltrates. Glomeruli are normal
Omeprazole was stopped. She was treated with 10 mg/kg of intravenous methyl prednisolone for 3 days followed by oral prednisolone 1 mg/kg tapered to 0.5 mg/kg over 3 months. Her renal parameters gradually improved, but she is left with residual renal impairment with serum creatinine of 1.6 mg % after 1 year.
Case 2
A 53-year-old female was admitted with a history of vomiting, loin pain for the 2 weeks and oliguria for 4 days. She was a known case of pulmonary tuberculosis who was receiving anti-tuberculosis therapy for the past 2 months. Pantoprazole was started for nausea 3 weeks ago. On examination, she appeared sick, febrile (100.5°F), tachypneic with a blood pressure of 130/80 mmHg. Blood urea was 76 mg/dl, serum creatinine 4.9 mg/dl and serum bicarbonate 14 mEq/L. She was started on daily hemodialysis for 4 days after which her general condition improved. Renal biopsy revealed AIN with dense lymphocyte and eosinophilic infiltrates in the interstitium [Figure 2]. PPI was stopped and she was started on oral corticosteroids for 8 weeks with continuation of anti-tuberculosis therapy. After 4 months her serum creatinine remained high at 1.6 mg/dl. Rifampicin induced AIN was considered unlikely given the temporal profile of events after start of pantoprazole and the fact that renal function rapidly recovered, after withdrawal of the latter with steroid therapy.

- Renal biopsy showing dense interstitial infiltrates with lymphocytes and scattered eosinophils. Some tubules show injury pattern
Case 3
A 22-year-old male was admitted with history of oliguria and back pain of 1-week duration. He had received pantoprazole and paracetamol for 10 days as treatment for dyspepsia and body ache. On examination, there was no fever, BP was 140/80 mm Hg, blood urea was 123 mg/dl, and serum creatinine 10.7 mg/dl. Hemodialysis was started. Renal biopsy showed AIN with lymphocytic and eosinophilic infiltrations in the interstitium with normal appearing glomeruli. PPI was stopped and he was started on pulse methyl prednisolone for 3 days followed by oral prednisolone for 12 weeks. His renal function normalized after 8 weeks and remained at 0.9 mg /dl after 12 months.
Case 4
A 68-year-old female was admitted with bilateral loin pain and recurrent vomiting for 1 week. She was prescribed esomeprazole for dyspepsia 3 weeks ago. She was afebrile, mildly volume depleted with a BP of 100/70 mmHg Intravenous saline was started. Serum creatinine was 2.6 mg/dl with trace proteinuria and pyuria. Renal biopsy showed AIN with dense interstitial infiltrates of lymphocytes, plasma cells, and eosinophils. Urine culture grew E. coli which was treated by antibiotics. PPI was stopped and oral prednisolone was started on 1 mg/kg dose for 8 weeks, which resulted in excellent recovery of her renal function. Her serum creatinine stabilized at 1.2 mg/dl at 4 months.
Discussion
Every year millions of prescriptions are made out for PPI to treat dyspepsia and peptic ulcer disease. PPI-induced AIN is thought to be a common class effect. It is triggered by a hypersensitivity immune reaction to the drug or one of its metabolites. Ruffenach et al., published the first case report of AIN due to omeprazole in 1992.[1] Since, then reports have emanated from many national adverse drug registries. World Health Organization adverse drug reaction report included 498 cases as of July 2011.[2] A single-center retrospective analysis was carried out of renal biopsy results of 296 consecutive patients between 1995 and 1999 from UK. Acute tubulointerstitial nephritis (TIN) was identified in 24 (8.1%) biopsies. Eight out of 14 cases with presumed drug related AIN could be attributed to the PPI such as omeprazole and lansoprazole.[3] PPI induced AIN is a generally disease of the middle or older age, which mirrors the symptom of dyspepsia in the aged. Median age in our cases was 52.5±18 years. Male; female ratio is 1:3 whereas there is equal sex predilection in other series. Pantoprazole was the commonest PPI in our cases. Omeprazole and esomeprazole were consumed by one each of our cases. In Myer's series of omeprazole induced AIN the drug was taken for an average 2.7 months before the onset of renal involvement.[4] Our case series differs in that the PPI has been ingested for short period of 1-8 weeks before the onset of AIN. The signs and symptoms off PPI-induced AIN were non-specific such as nausea, vomiting, loin pain, or fever. These cases highlight the complexity of diagnosis of PPI induced AIN. As can be seen from the Table 1 PPIs are rarely prescribed singly. Co-prescription with drugs which themselves are linked to AIN such as penicillin, cephalosporine and non steroidal anti inflammatory drugs is common. In case 2, even though anti-tuberculosis therapy was started a few months earlier, the symptoms were of shorter duration. Rifampicin which is well-known to produce AIN was continued through the illness with subsequent renal recovery proving that this was not the cause of disease. None of our patients had fever, skin rash or joint pains. Rossert encountered these only in <5% of drug induced AIN.[5] Hence, without a renal biopsy correct diagnosis is likely to be missed.

Inactive urinary sediment in the absence of significant hematuria or proteinuria should make one suspect AIN. None of these cases had eosinophilia. The severity of renal failure at presentation varied. Two of them had severe renal failure while the rest had mild to moderate renal failure. All of them showed almost uniform renal biopsy findings of extensive lymphoplasmacytic infiltrations involving the interstitium with sparing of the glomeruli. Eosinophils were seen in only two cases. Out of 18 renal biopsies reported by Geevasinga et al., classic picture of AIN with eosinophils emerged in 82%.[2]
We stopped the PPI in all cases and based on the severity of interstitial infiltrations either pulse methyl prednisolone or oral prednisolone was started. Hemodialysis was required in two cases of severe acute renal failure. Four patients in the Torpey's case series presented to emergency room with acute renal failure.[3] We continued prednisolone for a period of 8-12 weeks. In a series from New Zealand, 12 out of 14 cases of PPI induced AIN were left with permanent renal impairment.[6] In published case series, steroid therapy was required in many cases with good recovery of renal function.[78] At variance is a report from pharmacovigilance laboratory of Netherlands, wherein six out of seven cases recovered renal function once PPI was stopped.[9] Even with early management, two of our patients were left with significant renal impairment. Permanent dialysis dependency may be encountered especially in diabetics. Indian population may have racial predisposition to AIN as shown in a study.[10] Polymorphisms in hepatic and renal CYP450 microsomal enzyme system may underlie susceptibility to drug toxicity by increasing intra renal levels of the drug or its metabolites.[1112]
The implications of our findings are important both for the physicians and general public at large in India where over the counter prescriptions of drugs for upper gastrointestinal symptoms constitute second largest drug market share equivalent to 231 million US dollars.[13] Thus, even uncommon side-effects such as PPI induced AIN will be encountered regularly by the physicians given the sheer size of drug usage. Out-patients should be educated by the physicians to return if they develop loin pain and oliguria. On the other hand, PPI induced AIN should be considered in any in-patient who develops hospital acquired acute kidney injury.
Conclusion
PPI induced AIN is likely to be under recognized and undertreated in India. Its symptoms are non-specific. A high index of suspicion about this condition should prompt the physician to stop the drug, perform a renal biopsy if needed and start steroid therapy for halting a progressive renal disease.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4:597-604.
- [Google Scholar]
- Drug-induced tubulo-interstitial nephritis secondary to proton pump inhibitors: Experience from a single UK renal unit. Nephrol Dial Transplant. 2004;19:1441-6.
- [Google Scholar]
- Acute interstitial nephritis due to omeprazole. Am J Gastroenterol. 2001;96:3428-31.
- [Google Scholar]
- Proton pump inhibitors and acute interstitial nephritis: Report and analysis of 15 cases. Nephrology (Carlton). 2006;11:381-5.
- [Google Scholar]
- Drug associated acute interstitial nephritis: Clinical and pathological features and the response to high dose steroid therapy. Q J Med. 1983;52:194-211.
- [Google Scholar]
- Evaluation of clinical and histological prognostic markers in drug-induced acute interstitial nephritis. Ren Fail. 1996;18:97-104.
- [Google Scholar]
- Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol. 2007;64:819-23.
- [Google Scholar]
- The diagnosis and racial origin of 394 patients undergoing renal biopsy: An association between Indian race and interstitial nephritis. Nephrol Dial Transplant. 1997;12:71-7.
- [Google Scholar]
- Proton pump inhibitors and the kidney: Critical review. Clin Nephrol. 2007;68:65-72.
- [Google Scholar]
- Organisation of Pharmaceutical Producers of India Mumbai. Available from: http://www.indiaoppi.com
- [Google Scholar]







