Translate this page into:
Acute kidney injury and mortality in hematopoietic stem cell transplantation: A single-center experience
Address for correspondence: Dr. B. Sehgal, Department of Nephrology, Christian Medical College and Hospital, Ludhiana, Punjab, India. E-mail: blessy.sehgal@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hematopoietic stem cell transplant (HSCT) is a life-saving procedure for patients with several malignant and nonmalignant hematological disorders. Acute kidney injury (AKI) is a common complication after HSCT. The aim of the study was to identify the incidence and outcomes of AKI associated with HSCT in our center. Sixty-six HSCT recipients from October 2008 to March 2014 at Christian Medical College, Ludhiana, were followed up till July 31, 2014. RIFLE criteria utilizing serum creatinine was used to diagnose and stage AKI. Mortality and AKI were the primary outcomes studied. The risk of AKI in relation to conditioning regimen, type of HSCT (allogeneic and autologous), co-morbidities, graft versus host disease, drug toxicity, and veno-occlusive disease were analyzed. Sixty-five patients were included in the study. Male: Female ratio was 3.6:1 with a median age of 17 years (1.5–62). Forty-nine (75.4%) patients had AKI over 3 months, R 17 (26.2%), I 19 (29.2%), and F 13 (20%). AKI occurred at a mean of 19.4 ± 29.2 days after the HSCT. AKI was more commonly observed in patients undergoing allogeneic versus autologous HSCT (85.2% in allogeneic vs. 27.8% in autologous, P = 0.005). Mortality was seen in 20 patients (30.8%) in 3 months. AKI in the first 2 weeks (P < 0.016) was a significant risk factor for mortality. Incidence of AKI in HSCT is high and accounts for significant mortality and morbidity. RIFLE classification of AKI has prognostic significance among HSCT patients with an incremental trend in mortality.
Keywords
Acute kidney injury
hematopoietic stem cell transplant
RIFLE criteria
Introduction
Hematopoietic stem cell transplantation (HSCT) is done for malignant (multiple myeloma, leukemia, and lymphomas) and nonmalignant (aplastic anemia, beta-thalassaemia, immunodeficiency disorders, and inborn errors of metabolism) hematological disorders, and some solid tumors, which otherwise are incurable.[1] It involves administration of high-dose chemotherapy or chemo-radiotherapy as a conditioning regimen to destroy tumor cells and simultaneously suppress the host's immune system to prevent graft rejection. The different conditioning regimens used are myeloablative (MA), non-MA (NMA), and reduced intensity conditioning (RIC). MA HSCT uses maximally tolerated dose of total body irradiation, with or without chemotherapy, or chemotherapy alone. This results in profound pancytopenia within 1–3 weeks from administration, which is usually irreversible and in most instances fatal, unless hematopoiesis is restored by hematopoietic stem cell infusion. On the contrary, non-MA HSCT relies more on donor cellular immune effects and less on the cytotoxic effects of the preparative regimen to control the underlying disease. Here, prompt hematopoietic recovery occurs (<28 days) without stem cell support.[2] RIC regimens do not fit the criteria for MA or NMA regimens: they cause cytopenia of variable duration, and should be given with stem cell support, although cytopenia may not be irreversible.[3]
Despite advances in supportive care, transplant-related organ dysfunction is a major contributor to transplant-related complications with acute kidney injury (AKI) being one of the most severe of these toxicities. Various studies have shown an incidence ranging from 23% to 53% according to the different cut-offs used to define AKI.[4] AKI frequently develops together with other posttransplant complications such as graft versus host disease (GVHD), respiratory infections, drug-related toxicities, and co-morbidities, leading to a multi-organ dysfunction syndrome in a high number of these patients.[5] Occurrence of AKI in post-HSCT period is associated with a high morbidity and mortality, with studies reporting a high mortality rate ranging from 75% to 100% in patients developing AKI requiring dialysis for treatment.[6] A major problem encountered while drawing inferences from these studies was the inconsistencies in the definition used to classify AKI, which is a major problem in AKI research. The consensus definition for AKI using the RIFLE criteria based on changes in the patients' creatinine and/or urine output has enabled standardization of diagnosis and care of patients with AKI.[7]
We report our experience in a cohort of patients who underwent HSCT in a single center. We analyzed AKI incidence based on RIFLE criteria and risk factors, and also the impact of developing AKI on outcome.
Subjects and Methods
Patient selection
We included 66 consecutive adult and pediatric patients who underwent HSCT in our institution between October 2008 to March 2014. These patients underwent transplant for hematological malignancies as well as nonmalignant hematological diseases. Data on patient demographics and clinical course were collected on all patients. Patients with diagnosed chronic kidney disease (CKD) diagnosed on the basis of KDOQI definition were excluded from the analysis.[8] One patient with CKD was excluded and 65 patients were analyzed.
Conditioning regimen
We used standard conditioning regimens depending on the disease. Briefly, fludarabine-busulfan (Flu-40 mg/m2 and Bu-130 mg/m2) in acute myeloid leukemia/myelodysplastic syndrome; melphalan (Mel-180-200 mg/m2) in multiple myeloma; busulfan-cyclophosphamide in ALL (Bu-0.8 mg/kg and Cy 60 mg/kg); fludarabine-cyclophosphamide in aplastic anemia (Flu-40 mg/m2 and Cy 60 mg/kg); and treosulfan-fludarabine-thiotepa for patients with thalassaemia major (Treo 14 g/m2, Flu-40 mg/m2 and thiotepa-8 mg/kg).[9] In allogeneic transplantation, bone marrow and peripheral stem cells from related donors were used as the source of hematopoietic progenitors. In some patients, cryopreserved umbilical cord blood and matched unrelated donors were also used as the source of hematopoietic progenitor cells. Standard prophylaxis against Pneumocystis carinii, fungal infections, and cytomegalovirus (CMV) was used.[10] Broad-spectrum antibiotics were used for febrile neutropenia as per the institutional protocol. Antibiotics included cefepime or piperacillin-tazobactam followed by the addition of teicoplanin/vancomycin if febrile, followed by carbapenem replacing the first line broad-spectrum antibiotics, if fever persisted. Amikacin was used alone or in combination in 14 patients. If the fever persisted for more than 48–72 h, addition of an antifungal agent was considered as per the protocol. Amphotericin or voriconazole was mostly used.
Graft versus host disease prophylaxis
The patients undergoing allogeneic HSCT were initiated on GVHD prophylaxis with cyclosporine (53 patients)/tacrolimus (1 patient) and a short course methotrexate. The cyclosporine dose was adjusted to trough levels between 150 and 300 ng/ml. Methotrexate (MTX) was administered on days + 1, +3, +6, and + 11 days (10 mg/m2) with folinic acid rescue.
Patient monitoring
Demographic characteristics were collected at the time of HSCT. Gender, age, diagnosis, and underlying disease were recorded for all patients. The following complications were observed hypotension (systolic blood pressure less 90 mm of Hg), acute GVHD, sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) of liver,[11] bacterial sepsis, CMV infection, and probable fungal infection. Use of nephrotoxic drugs and inotropes was recorded. In the present study, sepsis diagnosis is based on temperature >38°C or <36°C, white blood cell count >10,000/mm3 or <4000/mm3, and positive blood culture for bacteria.[12]
Clinical diagnosis of SOS/VOD was established according to clinical criteria when two of the following events occurred within 30 days after transplantation: hyperbilirubinemia, hepatomegaly, right upper quadrant pain of liver origin, and sudden unexplained weight gain (>2% of baseline body weight).[11] GVHD was diagnosed and graded according to the established criteria.[13] During the posttransplant course, patients were followed up till July 31, 2014. Primary outcome of this study was AKI. Survival of patients at day 90 and last follow-up were also noted.
Identification of acute kidney injury
The severity of AKI was classified according to the RIFLE criteria. Using the RIFLE criteria (acronym indicating Risk of renal dysfunction; Injury to the kidney; Failure of kidney function; Loss of kidney function and end-stage kidney disease), AKI was classified into three categories based on severity (risk, injury, and failure) and two categories based on clinical outcome (Loss and end-stage kidney disease). The outcome RIFLE categories loss and end-stage kidney disease were not evaluated in this study. The criteria for classification of AKI-R, AKI-I, and AKI-F are as follows: AKI-R was considered if there was an increase in serum creatinine to more than 1.5-fold of baseline value or a urinary output lower than 0.5 ml/kg/h for 6 h. AKI-I was considered if there was an increase in serum creatinine to more than 2-fold of baseline value or urinary output lower than 0.5 ml/kg/h for 12 h. AKI-F was considered if there was an increase in serum creatinine to more than 3-fold baseline value or urinary output lower than 0.3 ml/kg/h for 24 h. Because other confounding factors may influence urine output, in the present study, patients were assigned to RIFLE categories only according to serum creatinine criteria.[6]
Statistical analyses
Continuous variables were expressed as mean ± standard deviation and compared by the F test. The categorical variables were expressed as percentages and compared by the Chi-square test or Fisher's exact test. The Kaplan–Meier method was used to calculate time to death for AKI. The survival curves were compared using the log-rank test. All P values were two-tailed, and a value of ≤ 0.05 was considered statistically significant. SPSS version 16.0 statistical software was used to perform the analysis
Results
Baseline characteristics
A total of 66 recipients underwent transplantation during the study period. One patient was excluded from the study as he had CKD prior to transplant. Sixty-five patients were included for data analysis, 51 males and 14 females with M: F ratio of 3.6:1. The median age was 17 years ranging from 1.5 to 62 years. The mean pre HSCT creatinine was 0.53 ± 0.24 mg/dL. Among the 65 patients, 54 (83.1%) underwent allogeneic and 11 (16.9%) autologous HSCT. The diseases for which transplant was done included both malignant and nonmalignant hematological diseases. Co-morbidities were observed in 39 (60%) patients, iron overload being the most common seen in 19 (28.8%) patients [Table 1].

Acute kidney injury stratified by the RIFLE criteria
Out of the 65 patients, 31 (47.7%) developed AKI over 2 weeks and the number increased to 49 (75.4%) patients by the end of 3 months post-HSCT. AKI occurred at a mean of 19.4 ± 29.2 days and median 9 (0–150) days after the HSCT. A total of 99 episodes of AKI were seen in the 49 patients with 19 patients having single episode of AKI, 17 developing two episodes, 8 patients developing three episodes, 3 patients developing four episodes, and 2 patients developing five episodes. AKI was observed with risk (R) in 15 (23.1%), injury (I) in 11 (16.9%), and failure (F) in 5 (7.7%) over 2 weeks and 49 (75.4%) patients had AKI over 3 months with R in 17 (26.2%), I in 19 (29.2%), and F in 13 (20%) of the patients [Figure 1]. Four patients (6.1%) with AKI needed dialysis.

- Period prevalence of acute kidney injury stratified according to RIFLE criteria
Risk factors for acute kidney injury
There were no statistical differences in age, gender, or primary disease among the various classes of AKI. Univariate analysis demonstrated that the major factors associated with AKI over 3 months were nephrotoxic drug use and requirement of inotropes, acute GVHD, SOS/VOD, and probable fungal infection [Table 2]. Nephrotoxic drug use was seen in a total of 38 (58.5%) of patients, with amphotericin being the most common nephrotoxic drug administered in 25 (65.8%) of them. There was an incremental trend noted in the nephrotoxic drug use and the grade of AKI. Inotropes were used in 15 (23.1%) patients, among whom all the patients developed AKI with an incremental increase with grade of AKI. Acute SOS/VOD was seen in 7 (10.8%) patients. All the patients who had SOS/VOD developed AKI (P = 0.004). GVHD was observed in 26 (40%) of the patients and occurred at median of 32 days with range from as early as 6 days to 115 days. The incidence of AKI-I and AKI-F in patients with GVHD was significantly higher than in patients without GVHD (P = 0.009). Sepsis, CMV infection, and CSA levels were not associated with increased risk of developing AKI [Table 2].
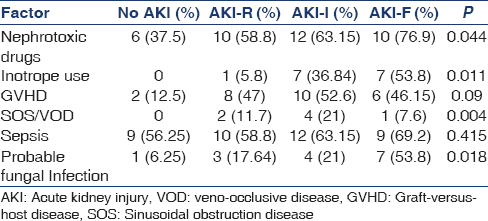
Allogeneic HSCT (odds ratio [OR]: 10.5 [95% confidence interval [CI]: 2.02–45.5]), ionotrope drug use (OR: 4.48 [95% CI: 3.67–13.8]) and probable fungal infection OR 3.26 [95% CI: 1.27–28.4]) were significant risk factors for developing AKI over 3 months on multivariate analysis.
Survival analyses
A high mortality was associated with patients undergoing HSCT who developed AKI. 20 patients (30.8%) died during the first 3 months. Death rates increased with worsening AKI. Death occurred in 6 (17.6%) patients without AKI, 6 (40%) with AKI graded as risk (R), 4 (36.4%) with injury (I), and in 4 (80%) with failure F (P = 0.027). Univariate analysis showed that death was associated with AKI over the first 2 weeks, inotropic drug use, GVHD, high CSA trough levels, and fungal infection. A statistically significant difference was seen in trough cyclosporine levels between the patients who died and those who survived [Table 3].

Multivariate analysis showed that early AKI (over first 2 weeks) was an independent risk factor for death (OR: 1.567 [95% CI: 1.34–1.79]) [Figure 2], other significant factors included ionotrope use OR 6.97 (95% CI: 4.13–14.67), probable fungal infection (OR: 7.01 [95% CI: 2.47–14.32]), and high CSA trough levels [OR]: 6.071 [95% CI: 1.89–13.8]).

- Acute kidney injury associated with the increased mortality of patients in the 90 days posttransplant period
Discussion
HSCT provides effective therapy and a potential cure for patients with a number of diseases. The major indications include hematological malignancies such as acute myeloid leukemia, acute lymphoid leukemia, and chronic myeloid leukemia in advanced phase, as well as marrow failure states such as aplastic anemia and genetic disorders such as b-thalassemia. Worldwide, approximately, 40,000–50,000 transplantations are performed annually, and the number continues to increase by 10–20% each year.[14] In addition to other transplant-related organ toxicities, occurrence of AKI is a common complication associated with HSCT. The majority of AKI were seen in the first 100 days posttransplant.[15] In this study, 31 (47.7%) patients developed AKI over first 2 weeks and AKI developed in 49 (75.4%) patients over 3 months with R in 17 (26.2%), I in 19 (29.2%), and F in 13 (20%) of the patients similar to a study by Bao et al. which showed that 48.9% of allo-HSCT patients have various degrees of AKI, including 40 patients (28%) in AKI-R, 18 (12.6%) in AKI-I, and 12 (8.4%) in AKI-F.[16] AKI occurs commonly in the early posttransplant period. RIFLE criteria has the advantage of early and accurate diagnosis of AKI. In this study, it occurred at a median of 9 (0–150) days after the HSCT compared to a study by Saddadi et al., which also showed an early occurrence of AKI with the median time to AKI after HSCT was 18 days (range, 1–185 days).[17] Previously done studies have shown that AKI post-HSCT is multifactorial, various causes include tumor lysis syndrome, sepsis, nephrotoxic drugs, hepatic sinusoidal obstruction/veno-occlusive disease, and thrombotic microangiopathy.[18]
AKI was found to be 85.18% among those undergoing allogeneic transplant as compared to 27.27% in those undergoing autologous transplant. In a single center, retrospective study done at the Hospital de Santa Maria in Lisboa, Portugal, which included 140 patients who underwent HSCT and they found that the incidence of AKI in recipients of allogeneic HCT was 27% compared to an incidence of 12% in those undergoing autologous HCT.[19] There is a marked difference in the incidence in the AKI reported in both the studies. The reasons could be multifactorial. This being one of the first reported studies from India, the exact reasons for this difference needs to be evaluated. The use of conventional amphotericin could be considered a probable cause. In a study done at the Bone Marrow Transplantation Unit of Istanbul School of Medicine, it was found that sepsis in the autologous HCT recipients and SOS/VOD, acute GVHD and cyclosporine toxicity in the allogeneic HCT recipients were the major risk factors for the development of AKI.[20]
SOS/VOD is a major risk factor and has shown to consistently predict AKI, especially in patients receiving MA conditioning. In our study, AKI developed in all the 7 patients who had SOS/VOD. It has been assumed that portal hypertension resulting from hepatic sinusoidal injury leads to both decreased renal perfusion and tubular injury ultimately leading to AKI.[21] Zager, found that up to 80% of patients with SOS/VOD developed AKI.[22]
Acute GVHD independently increases the risk for AKI.[6] Acute GVHD was observed in 26 (40%) of the patients and occurred at a median of 32 days with range from as early as 6 days to 115 days. The present study also showed that the incidence of all grades of AKI in patients with GVHD was significantly higher than in those without GVHD. Acute GVHD can contribute to AKI directly through cytokine- and immune-related injury, including glomerular deposits causing nephrotic syndrome and tubulitis or indirectly through nephrotoxicity induced by cyclosporine prophylaxis.[23] In addition, severe GVHD with diarrhea and dehydration and CMV reactivation because of immunosuppression with high-dose prednisolone can also contribute to GVHD-associated nephrotoxicity.[24] Another important risk factor for developing AKI in post-HSCT is the use of nephrotoxic drugs which include antibiotics such as aminoglycosides, colistin, and amphotericin. It was found in our study that there was an increase in incidence of AKI in patients who received nephrotoxic drugs as compared to those who did not (P = 0.04). Amphotericin B is directly toxic to the distal tubular epithelium. It also induces renal vasoconstriction and reduces renal blood flow and glomerular filtration rate.[18] Studies have demonstrated an increased occurrence of AKI in patients receiving conventional or liposomal preparations of amphotericin. In our study, amphotericin was the most common nephrotoxic drug used either alone or in combination with others. In a prospective, randomized controlled trial involving Intensive Care Unit patients with candidal infections, Sorkine et al. found that 66.7% of patients treated with conventional amphotericin experienced an increase in their serum creatinine.[25] A relationship based on conventional or liposomal amphotericin was difficult to conclude in our study because some patients were switched to conventional from liposomal amphotericin owing to financial constraints.
AKI is a risk factor for mortality and has been shown in a number of previously done studies. Zager et al. first noted the significant differences in mortality associated with varying degrees of AKI in transplantation. In patients without renal insufficiency, the mortality was 17% in patients with nondialysis-requiring renal failure, the mortality was 37% in patients with renal failure requiring dialysis, the mortality was 84%.[26] A meta-analysis encompassing 1211 patients undergoing MA allogeneic transplantation found that the relative risk of death after renal failure was greater than two-fold higher.[27] Similar results were found in our study with a death rate of 30.8% and an incremental risk of AKI grade and death. Increased mortality in AKI may be caused by volume overload, coagulation abnormalities, increased incidence of sepsis with multi-organ failure, and cytokine or immune-mediated major organ dysfunction. AKI can also interfere with dosing of immunosuppressive drugs including calcineurin inhibitors, and may lead to the development of GVHD, which can act in a vicious cycle to worsen AKI and increase mortality.[15] Therapies preventing and treating AKI may substantially improve patients' prognosis after HSCT. The RIFLE criteria appear to be sensitive to early changes in kidney function after HSCT and early diagnosis, taking adequate prophylactic measures, and controlling SOS/VOD and GVHD may prevent occurrence and development of AKI post-HSCT. Effective measures to diagnose and treat AKI early may improve the survival of patients after HSCT and decrease the mortality rate of patients after HSCT.
Limitations
This was a retrospective, single-center study which included a small cohort of patients. Owing to other confounding factors that could influence urine output, such as diuretic therapy, patients were assigned to RIFLE categories according to serum creatinine only.
Conclusions
AKI is a frequent complication following HSCT and the majority of cases occur within the first 3 months after transplantation. AKI is usually more severe in allogeneic HSCT than in the autologous HSCT. SOS/VOD, GVHD, nephrotoxic drug use, and need for inotropes were identified as risk factors for AKI. AKI was an independent prognostic factor for mortality after HSCT. Mortality in the first three months of HSCT increased with the severity of AKI categories. Early recognition of patients at risk for development of AKI post-HSCT and treatment is paramount to improve the outcomes of an otherwise life-saving treatment. The RIFLE criteria are an important tool to stratify these patients and an incremental and risk of death.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Advances in allogeneic stem cell transplantation: Directing graft-versus-leukemia at solid tumors. Cancer J. 2002;8:2-11.
- [Google Scholar]
- Differences between the different conditioning regimens for allogeneic stem cell transplantation. Curr Opin Oncol. 2006;18:667-70.
- [Google Scholar]
- Defining the intensity of conditioning regimens: Working definitions. Biol Blood Marrow Transplant. 2009;15:1628-33.
- [Google Scholar]
- Acute renal failure in patients following bone marrow transplantation: Prevalence, risk factors and outcome. Am J Nephrol. 1995;15:473-9.
- [Google Scholar]
- Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566-73.
- [Google Scholar]
- Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant. 2003;32:405-10.
- [Google Scholar]
- Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-12.
- [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266.
- [Google Scholar]
- Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: Results of a prospective multicentre study. Br J Haematol. 2001;115:653-9.
- [Google Scholar]
- Bacterial, fungal and protozoan infection after marrow transplantation. In: Recent Advances and Future Directions in Bone Marrow Transplantation: Experimental Hematology Today. Springer Link 1988:171-6.
- [Google Scholar]
- Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50:781-9.
- [Google Scholar]
- Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
- [Google Scholar]
- 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825-8.
- [Google Scholar]
- Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: A global observational study. Haematologica. 2013;98:1282-90.
- [Google Scholar]
- Acute kidney injury following HCT: Incidence, risk factors and outcome. Bone Marrow Transplant. 2011;46:1399-408.
- [Google Scholar]
- An evaluation of the RIFLE criteria for acute kidney injury after myeloablative allogeneic haematopoietic stem cell transplantation. Swiss Med Wkly. 2011;141:w13225.
- [Google Scholar]
- Frequency, risk factors, and outcome of acute kidney injury following bone marrow transplantation at Dr Shariati Hospital in Tehran. Iran J Kidney Dis. 2010;4:20-6.
- [Google Scholar]
- Acute renal failure after myeloablative hematopoietic cell transplant: Incidence and risk factors. Kidney Int. 2005;67:272-7.
- [Google Scholar]
- Acute renal failure following myeloablative autologous and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2006;38:707.
- [Google Scholar]
- Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2006;38:141-7.
- [Google Scholar]
- Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27-42.
- [Google Scholar]
- Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443-58.
- [Google Scholar]
- Postmortem examination of the kidney in allogeneic hematopoietic stem cell transplantation recipients: Possible involvement of graft-versus-host disease. Int J Hematol. 2008;87:225-30.
- [Google Scholar]
- Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol Blood Marrow Transplant. 2008;14:125-31.
- [Google Scholar]
- Administration of amphotericin B in lipid emulsion decreases nephrotoxicity: Results of a prospective, randomized, controlled study in critically ill patients. Crit Care Med. 1996;24:1311-5.
- [Google Scholar]
- Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J Kidney Dis. 1989;13:210-6.
- [Google Scholar]
- Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005;67:1999-2005.
- [Google Scholar]







