Translate this page into:
Acute Kidney Injury in Paraquat Poisoning : A Study of 400 Cases
Corresponding author: Manjusha Yadla, Department of Nephrology, Gandhi Medical College, Hyderabad, India. E-mail: manjuyadla@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadla M, Bonthu S, Burri SB, Bakka VK, Pathakala S. Acute Kidney Injury in Paraquat Poisoning : A Study of 400 Cases. Indian J Nephrol. doi: 10.25259/IJN_712_2024
Abstract
Background
Paraquat (PQ) a synthetic weedicide is often consumed with the intent to commit suicide. PQ can accumulate in the lungs and kidneys through redox reactions, generating reactive oxygen species (ROS) and causing severe pulmonary fibrosis and acute tubular necrosis. Literature on PQ-acute kidney injury (PQ-AKI) is limited.
Materials and Methods
We reviewed data on patients referred to nephrology services to manage PQ-AKI between June 2014 and June 2024. We analyzed epidemiological data, clinical features, and outcomes.
Results
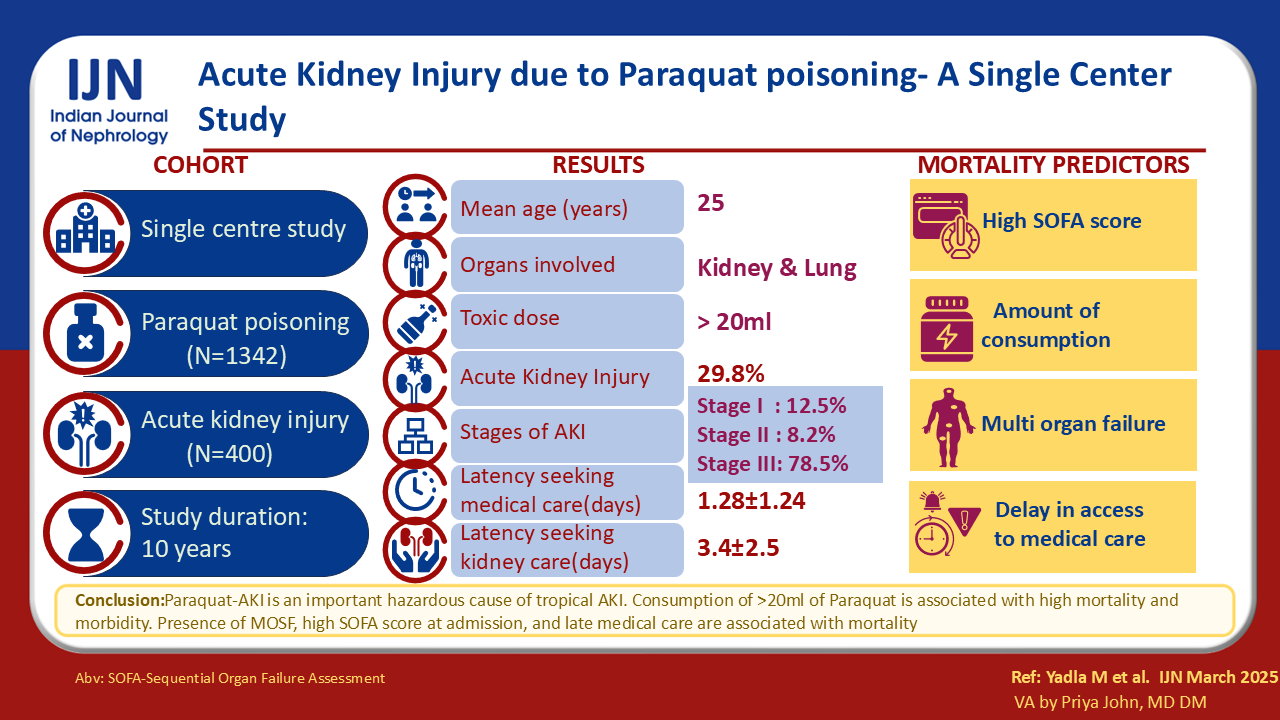
Four hundred patients were analyzed. The mean age was 30 ± 11 years. Of 1342 PQ admissions, approximately 30% developed AKI, with PQ-AKI accounting for 6. 2% of all cases with AKI during the period. Oligoanuria and deranged kidney function were reasons for referral. The majority were in stage 3 AKI (75%), of whom 45% received hemodialysis (HD). The mortality rate (75%) was associated with consumption quantity, gender, and multiorgan failure.
Conclusion
PQ-AKI is an important contributor to AKI in this region and is associated with high mortality. Quantity of consumption, gender, multiorgan failure, and latency in seeking medical care were associated with outcomes.
Keywords
AKI
India
Mortality
Paraquat
Tropical AKI
Introduction
Paraquat (PQ), N, N’-dimethyl-4, 4′-bipyridinium dichloride, is a synthetic, quaternary, nitrogenous, organic compound.1 It is a non-selective contact pesticide that also destroys weeds by forming superoxide anions during photosynthesis. PQ poisoning is becoming a serious public health concern, especially in developing countries, due to easy access and lack of awareness of potential harm. Exposure can occur accidentally or intentionally. PQ accumulates in the lungs and forms free radicals causing lipid peroxidation. NADPH gets depleted, causing diffuse alveolitis and extensive pulmonary fibrosis, leading to respiratory failure. Severe poisoning affects the liver, kidneys, and lungs, and respiratory failure primarily causes death.2
Decreased absorption by gastric lavage and oral activated charcoal; elimination by conventional hemodialysis (HD), hemoperfusion (HP), continuous veno-venous hemofiltration, and continuous renal replacement therapy (CRRT); decreasing inflammatory response using immunosuppressants (corticosteroids and cyclophosphamide); antioxidants (N-acetyl cysteine, Vitamin C); and a combination of therapies were used to treat patients with PQ intoxication with varying outcomes.3-5
PQ is one of the important causes of tropical acute kidney injury (AKI). This compound is readily available and commonly used in India. PQ-AKI is associated with high morbidity and mortality rates. However, the literature is limited to case reports and case series.6 We undertook this study to analyze the clinical features and outcomes of PQ poisoning.
Materials and Methods
This is a ten-year retrospective review of records of patients admitted to nephrology services for PQ-AKI management between 2014 and 2024 at Gandhi Hospital, Hyderabad, Telangana. AKI was defined according to definitions given by KDIGO, 2012.7
PQ poisoning was diagnosed based on an alleged history of consumption and clinical presentations. We did not estimate PQ blood levels or test for urine dithionate positivity. PQ poisoning was classified based on consumed amounts as “mild” if the consumption was < 20 mL, “moderate” if 20-40 mL, and “high” if >40mL.
Patients were treated with gastric lavage, followed by oral steroids, N acetylcysteine, and vitamin C. Dialysis was given as indicated for patients with AKI. We obtained the Institute Ethics Committee’s approval and patient consent was obtained. We assessed demographic details, latency of referral, lab parameters, mode of renal replacement therapy, kidney injury outcomes, and factors affecting mortality.
Patients’ baseline characteristics, presumed causes, clinical presentation, outcomes, and management were analyzed using descriptive statistics. The data were presented as patients’ number (%), mean and standard deviations (SD). Factors potentially associated with death were analyzed using the t-test for continuous variables, Chi-square test, or Fisher’s exact test. We examined the variables using multivariate logistic regression to predict mortality. The analyses were conducted using SPSS software version 28.0, and p-values < 0.05 were considered statistically significant.
Results
The study included 400 patients with PQ-AKI. The study age range was 6 to 70 years, averaging 30.7 ± 11.1 years. Suicide and inadvertent consumption account for 90% and 10% of PQ poisoning cases, respectively. Amongst accidental ingestion cases, alcohol intoxication mostly led to PQ consumption. Quantity of consumption was mild in 53%, moderate in 30%, and high in 16% of patients [Table 1]. Most typically, PQ was consumed with soft drinks/alcohol. PQ is a readily available and unrestricted weedicide. The mean latency in seeking medical care and nephrology referral was 1.28 ± 1.24 and 3.4 ± 2.5 days, respectively.
| Baseline characteristics | |
|---|---|
| Age (years) | 30.77 ± 11.16 |
| Sex | |
| Males | 300 (75%) |
| Females | 100 (25%) |
| Amount of PQ | |
| Mild | 212 (53%) |
| Moderate | 122 (30.5%) |
| High | 66 (16.5%) |
| Reason for consumption | |
| Suicidal | 361 (90.3%) |
| Latency | |
| Latency to first medical care (days) | 1.28 ± 1.24 |
| Latency of nephrology referral (days) | 3.24 ± 2.5 |
| Clinical presentation | |
| Vomiting | 350 (87.5%) |
| Oral ulcers | 253 (63.25%) |
| Oliguria | 162 (40.5%) |
| Pain abdomen | 125 (31.2%) |
| Jaundice | 117 (29.2%) |
| Breathlessness | 104 (26%) |
| Dysphagia | 65 (16.2%) |
| Malena | 24 (6%) |
| Indication of KRT | |
| Metabolic acidosis | 81 (20.7%) |
| Raised renal parameters | 126 (31.5%) |
| Renal symptoms | |
| Anuria | 21 (5.38%) |
| Oliguria | 162 (41.5%) |
| Nonoliguria | 207 (53.07%) |
| AKI stage 1 | 53 (12.5%) |
| AKI stage 2 | 33 (8.2%) |
| AKI stage 3 | 314 (78.5%) |
| Mode of KRT | |
| Hemodialysis | 169 (42.2%) |
| CRRT | 79 (19.7%) |
| Peritoneal dialysis | 66 (16.5%) |
| Hemoperfusion | 76 (19%) |
| No KRT | 10 (2.5%) |
| Complication during RRT | 17 (4.2%) |
| Days of hospital stay | 6.9 ± 3.7 |
| Ventilatory support | 116 (29%) |
| Primary outcome | |
| Death | 295 (73.7%) |
| Survival | 105 (26.2%) |
| Secondary outcome | |
| Renal recovery (complete) | 93 (23.2%) |
| Renal recovery (partial) | 12 (3%) |
| Cause of death | |
| ARDS | 190 (47.5%) |
| Aspiration pneumonia | 1 (0.3%) |
| MODS | 102 (34.5%) |
| Sudden cardiac death | 2 (0.5%) |
KRT: Kidney replacement therapy, AKI: Acute kidney injury, CRRT: Continuous renal replacement therapy, ARDS: Acute respiratory distress syndrome, MODS: Multi organ dysfunction syndrome
Vomiting (87%) was the most typical gastrointestinal symptom, followed by abdominal discomfort (31%), oral ulcers (63%), jaundice (29%), oliguria (40%), and breathlessness (26%). Referral to nephrology was primarily due to oligoanuria (45%). KDIGO staging confirmed stage 1 AKI in 13%, stage 2 in 8%, and stage 3 in 78% of patients [Table 1].
Oliguria and raised renal parameters most frequent indications for kidney replacement therapy (KRT) (72%). HD (42%), followed by peritoneal dialysis (16%) were most used. However, since CRRT and HP were available for the last two years, both treatments were done in 19% of patients each.
The mean serum creatinine in our cohort was 5 ± 3 mg/dL [Table 2]. Raised liver function parameters with mild transaminitis and hyperbilirubinemia were noted in a few patients.
| Hemoglobin (gm/dL) | 11.6 ± 1.8 |
| Total leucocyte counts (per mm3) | 11998.8 ± 5013.7 |
| Serum creatinine (mg/dL) | 5.04 ± 3.4 |
| pO2 | 84.79 ± 15.41 |
| Total bilirubin (mg/dL) | 3.94 ± 4.25 |
| Direct bilirurbin (mg/dL) | 2.26 ± 2.49 |
| SGOT (U/L) | 92.08 ± 83.21 |
| SGPT (U/L) | 101.5 ± 87.94 |
SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase
The study’s primary outcome, mortality, was high (75%). Significant contributors to this were Acute respiratory distress syndrome and multi-organ involvement [Table 3]. Regarding secondary outcomes, 23% and 3% of patients had complete and partial renal recovery, respectively. We could not include the present study’s short-term/long-term follow-up details.
| Parameter | Survivors | Non-survivors | p-value |
|---|---|---|---|
| Age (years) | 31.5 ± 12 | 30.6 ± 10.9 | 0.483 |
| Sex | |||
| Males | 71 (67.6%) | 229 (77.6%) | 0.042* |
| Females | 34 (32.4%) | 66 (22.4%) | |
| Latency to first medical care (days) | 1.24 ± 1.11 | 1.31 ± 1.28 | 0.644 |
| Latency to referral (days) | 3.13 ± 1.91 | 3.28 ± 2.68 | 0.597 |
| Amount of PQ | 0.032* | ||
| Mild | 90 (42.46%) | 122 (57.54%) | |
| Moderate | 13 (10.7%) | 109 (89.3%) | |
| High | 0 | 66 (100%) | |
| Serum creatinine (mg/dL) | 5.6 ± 3.3 | 4.9 ± 3.5 | 0.088 |
| Hemoglobin | 11.5 ± 2 | 11.7 ± 1.8 | 0.368 |
| WBC (per mm3) | 11119.5 ± 5668.6 | 12307 ± 4735.1 | 0.059 |
| pH | 7.4 ± 0.1 | 7.4 ± 0.2 | 0.021* |
| pO2 | 89.8 ± 12.9 | 83.1 ± 15.9 | <0.001* |
| Total bilirubin (mg/dL) | 3 ± 3.4 | 4.3 ± 4.5 | 0.002* |
| Direct bilirubin (mg/dL) | 1.6 ± 2.1 | 2.5 ± 2.6 | <0.001* |
| SGOT (U/L) | 66.5 ± 58.5 | 101.3 ± 88.8 | <0.001* |
| SGPT (U/L) | 77.3 ± 83.2 | 110.2 ± 88.2 | 0.001* |
| Ventilatory support | 11 (10.5%) | 105 (35.6%) | <0.001* |
| KRT indication | <0.001* | ||
| Metabolic acidosis | 8 (10.5%) | 73 (30.5%) | |
| Oliguria | 45 (59.2%) | 115 (48.1%) | |
| Anuria | 1 (1.3%) | 20 (8.4%) | |
| Raised renal parameters | 22 (28.9%) | 31 (13%) | |
| Other system involvement | <0.001* | ||
| Liver | 27 (25.7%) | 48 (16.3%) | |
| Lungs + Liver | 32 (29.5%) | 200 (67.5%) | |
Univariate analysis for determinants of mortality revealed a statistically significant association between variables such as quantity of consumption, gender, severe acidosis, and multiorgan failure (p<0.05). The variables mentioned above were statistically significant in multivariate analysis [Table 4].
| Dependent variable | OR | p-value | 95 % CI | OR | p-value | 95 % CI |
|---|---|---|---|---|---|---|
| Latency of referral | 0.927 | 0.017 | 0.870 - 0.896 | -1.42 3 | .001 | -2.25 - -0.61 |
| pO2 | 0.971 | 0.002 | 0.953 - 0.989 | -6.742 | <0.001 | -1.16 - -3.34 |
| Abnormal liver tests | 0.857 | 0.141 | 0.698 - 1.053 | 1.270 | .009 | 0.32 - 2.23 |
OR: Odds ratio, CI: Confidence interval
HP was given to 19% of patients, and all admitted within 72 hours of consumption, followed by CRRT in case of multi-organ failure or conservative management in those without multi organ system failure (MOSF)/AKI and HD/PD in those with AKI. HP was given for four hours on the day of admission. The HP group showed better survival outcomes [Table 5].
| Hemoperfusion | No Hemoperfusion | p value | |
|---|---|---|---|
| Sex | |||
| Males | 60 (78.9%) | 240 (74.1%) | 0.377 |
| Females | 16 (21.1%) | 84 (25.9%) | |
| Latency to first medical care (days) | 0.96 ± 1.21 | 1.36 ± 1.23 | 0.010* |
| Latency to referral (days) | 2.21 ± 2.32 | 3.49 ± 2.48 | <0.001* |
| Serum creatinine (mg/dL) | 2.82 ± 2.57 | 5.56 ± 3.37 | <0.001* |
| Hemoglobin (gm/dL) | 11.4 ± 1.31 | 11.71 ± 1.93 | 0.188 |
| WBC (per mm3) | 12058.16 ± 3469.06 | 11984.85 ± 5318.43 | 0.909 |
| Potassium (mEq/L) | 4.08 ± 0.68 | 4.32 ± 0.76 | 0.010* |
| pO2 | 88.51 ± 14.76 | 83.91 ± 15.45 | 0.019* |
| Total bilirubin (mg/dL) | 1.97 ± 1.64 | 4.4 ± 4.54 | <0.001* |
| Direct bilirubin (mg/dL) | 1.09 ± 1.22 | 2.53 ± 2.63 | <0.001* |
| SGOT (U/L) | 63.61 ± 52.59 | 98.76 ± 87.61 | 0.001* |
| SGPT (U/L) | 68.97 ± 57.06 | 109.13 ± 92.13 | <0.001* |
| Alkaline phosphatase (IU/L) | 137.49 ± 110.71 | 141.02 ± 96.4 | 0.780 |
| Anion gap | 14.75 ± 3.92 | 15.06 ± 5.11 | 0.620 |
| Ventilatory support | 2 (2.6%) | 114 (35.2%) | <0.001* |
| Other system involvement | <0.001* | ||
| Liver | 4 (5.3%) | 71 (21.9%) | |
| Lungs | 0 (0%) | 18 (5.5%) | |
| Lungs + Liver | 68 (89.5%) | 164 (50%) | |
| Mortality | 54 (71.05%) | 241 (74.38%) | <0.001* |
WBC: White blood cellcount, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase
Discussion
The PQ-AKI incidence is 29% and 6.2% in all PQ and AKI admissions, respectively. Our study encompasses 400 PQ-AKI patients over 10 years. It is the largest in existing literature. The primary outcome, mortality, was high, like related to the severity of poisoning and delayed referrals. KRT strategy was selected based on hemodynamic stability. Most patients were in multi-organ failure, needing supportive systems at admission.
In our study, we gave HP within 72 hours of PQ consumption. Our findings are contrary to reports of HP within four hours of consumption. The lower AKI stages and CRRT following HP in survivors may have increased survival rates in the HP group. Hence, survival cannot be solely attributed to HP and needs future assessment for confirmation.
Literature regarding PQ-induced AKI predominantly comprises case reports and series.8,9 A diverse range of AKI incidence (50-100%) was documented among patients admitted with PQ toxicity. In the present study, we observed the incidence of AKI to be 29%. Our study represents the most extensive study conducted to date. We identified a latency period of 1.28 days before the initiation of medical intervention, a phenomenon similarly reported in the existing literature.10 The factors linked to mortality align closely with those delineated in the current body of literature.10-12
Wang et al.13 and Hsu et al.14 have shown that CRRT would decrease mortality in patients of paraquat poisoning. Hsu et al. from Taiwan showed that early HP (< 4 hours) decreased mortality in 209 patients of severe paraquat poisoning.14 However, Yeh et al. from Taiwan showed that early HP or multiple sessions of HP do not decrease mortality.15
The volume of distribution of PQ is 1.2-1.6 L/Kg. The mean distribution half-life of plasma PQ is 5 hours, and the elimination half-life is 84 hours. PQ gets redistributed to organs and tissues within 4-5 hours of consumption and 82% gets excreted unchanged through the kidney in the first 48 hours, further slowly gets eliminated over days-weeks.16-18
The lungs and kidneys are primarily affected. The lungs uptake 10-20 times more PQ. Energy-dependent uptake by type 1 and type 2 alveolar epithelial cells causes elevated concentrations of the compound. Furthermore, the proximal tubular epithelial cells actively secrete PQ causing elevated concentrations in the renal tissues. Moderate to high PQ consumption causes redox reactions, releasing reactive oxygen species (ROS), resulting in pulmonary fibrosis. The underlying mechanism of PQ-AKI is only partly elucidated. It is thought to be due to recurrent redox reactions causing significant proximal tubular. Excess ROS production causes lipid peroxidation, inflammation, and cell death. Consumption of substantial quantities can lead to severe hepatocellular necrosis, damaging the liver. Heightened superoxide dismutase activity and diminished glutathione may cause severe liver necrosis. Given that PQ is primarily eliminated by the kidney, accumulation may occur in those with AKI, contributing to multi-organ failure.
PQ remains approved for use under the Insecticides/ Pesticides Registered under section 9(3) of the Insecticides Act, 1968 for use in the Country (Jul 1, 2022). It is widely recognized that PQ poisoning leads to high death rates. Therefore, the Government of India might start the ban on use according to Section 27(2) of the Insecticides Act, 1968. The government of Odisha introduced a prohibition on PQ and its derivatives in October 2024.
The volume of ingestion, delay in pursuing medical intervention, and the occurrence of multiorgan failure are linked to increased mortality. The availability of PQ should be regulated under stringent restrictions. Efforts to enhance education and awareness of PQ’s toxicity and distribution methods should be intensified. If an alternative herbicide is accessible, the government may consider banning PQ.
Recent advances in PQ poisoning include anthrahydroquinone-2-6-disulfonate19, Edaravone20 whose definitive roles are yet to be established in large studies.
The study had limitations – serum or urine PQ levels could not be estimated for the severity of poisoning due to a lack of resources. Follow-up after discharge to assess the long-term effects of PQ poisoning could not be done, and renal biopsy was not done in patients due to logistic and legal challenges.
To conclude, AKI may manifest in approximately one-third of cases involving PQ intoxication. An alarming mortality rate of 75% is observed in instances of PQ-AKI.
Conflicts of interest
There are no conflicts of interest.
References
- Goldfrank’s Toxicologic Emergencies (9th ed). New York: McGraw Hill; 2011.
- Outcome of paraquat poisoning - a five year study. Indian J Nephrol. 2003;13:64.
- [CrossRef] [Google Scholar]
- Failure of haemoperfusion and hemodialysis to prevent death in paraquat poisoning: a retrospective review of 42 patients. Medical toxicology and adverse drug experience. 1988;3:64-71.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11:387-91.
- [PubMed] [Google Scholar]
- Effect of antioxidants on the outcome of therapy in paraquat-intoxicated patients. Trop. J. Pharm Res. 2011;10
- [CrossRef] [Google Scholar]
- Golden hours in severe paraquat poisoning-the role of early haemoperfusion therapy. J Clin Diagn Res. 2017;11:OC06-OC08.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter Suppl. 2012;2:1-138.
- [Google Scholar]
- Discernment scheme for paraquat poisoning: A five-year experience in Shiraz, Iran. World J Exp Med. 2017;7:31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paraquat poisoning: A retrospective study of 55 patients from a tertiary care center in Southern India. Indian J Crit Care Med: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2020;24:155.
- [CrossRef] [Google Scholar]
- Predicting mortality in paraquat poisoning through clinical findings, focusing on pulmonary and cardiovascular system disorders. J Pharm Policy and Pract. 2023;16:123.
- [Google Scholar]
- Paraquat poisoning: analysis of an uncommon cause of fatal poisoning from Manipal, South India. Toxicol Int. 2015;22:30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical features and prognosis of paraquat poisoning: a review of 41 cases. Int J Clin Exp Med. 2015;8:8122.
- [PubMed] [PubMed Central] [Google Scholar]
- Time-dependent haemoperfusion after acute paraquat poisoning. Sci Rep. 2017;7:2239.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One. 2012;7:e48397.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Does hemoperfusion increase survival in acute paraquat poisoning? A retrospective multicenter study. Toxics. 2020;8:84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Toxicokinetics of paraquat in humans. Hum Exp Toxicol. 1990;9:5-12.
- [CrossRef] [PubMed] [Google Scholar]
- Paraquat pharmacokinetics in primates and extrapolation to humans. Toxicol Appl Pharmacol. 2021;417:115463.
- [CrossRef] [PubMed] [Google Scholar]
- Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anthrahydroquinone-2-6-disulfonate is a novel, powerful antidote for paraquat poisoning. Sci Rep. 2021;11:20159.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Retrospective study of clinical features and prognosis of edaravone in the treatment of paraquat poisoning. Medicine. 2019;98:e15441.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







