Translate this page into:
Acute Kidney Injury in Patients with Chronic Liver Disease: A Review
Corresponding author: Mohamed Hassanein, Division of Nephrology, Department of Medicine, University of Mississippi Medical Center, Jackson, United States. E-mail: mhassanein@umc.edu
-
Received: ,
Accepted: ,
How to cite this article: Elom HA, Hegazy Y, Lerma EV, Hassanein M. Acute Kidney Injury in Patients with Chronic Liver Disease: A Review. Indian J Nephrol. 2025;35:21-8. doi: 10.25259/ijn_333_23
Abstract
Introduction:
Acute kidney injury (AKI) is a frequent complication of chronic liver disease (CLD) contributing to high morbidity and mortality worldwide. While liver transplantation (LT) has shown favorable outcomes, early identification and management of AKI is imperative for survival. This review aims to highlight the epidemiology, pathophysiology, management, and prognosis of AKI in CLD.
Methods:
An extensive literature search was performed using PubMed, Medline, and Google Scholar to identify literature related to epidemiology, burden, clinical presentations, prognosis, and management of AKI in CLD.
Results:
The identified studies highlighted a wide range of prevalence of AKI in hospitalized patients with CLD. The etiology and pathophysiology are multifactorial and include prerenal AKI, acute tubular injury, sepsis, gastrointestinal bleeding, bacterial translocation from the gut, and hepatorenal syndrome (HRS). AKI is associated with a higher risk of morbidity and mortality and progression to chronic kidney disease following LT. Management of AKI in CLD varies based on the underlying etiology. While vasoconstrictors like terlipressin have shown great potential in the treatment of HRS-AKI and is widely used in Europe and United States, LT remains the definitive therapy of choice. In most cases, kidney replacement therapy serves as a bridge to liver transplant.
Conclusion:
AKI is a serious complication of CLD and early identification is essential. Diagnosis and management, particularly HRS is challenging and requires a high index of suspicion. More research is required to identify novel therapies to improve outcomes of AKI in patients with CLD.
Keywords
Acute kidney injury
Chronic liver disease
Hepatorenal syndrome
Kidney replacement therapy
Liver transplantation
Introduction
Chronic liver disease (CLD) is a major cause of morbidity and mortality worldwide.1 Approximately 2 million people die from liver diseases annually, accounting for 4% of all deaths worldwide.2 CLD is a spectrum of liver diseases marked by gradual distortion of liver architecture over a period of time, ultimately leading to cirrhosis or hepatocellular carcinoma (HCC). Complications of cirrhosis and HCC are predominantly the leading causes of all deaths from liver diseases.3 Over the years, the global etiology of CLD has shifted from viral hepatitis to metabolic dysfunction–associated steatotic disease (MASLD) previously known as non-alcoholic fatty liver disease (NAFLD).4 This is notably due to the rising incidence of obesity and improved prevention and treatment of viral hepatitis.
The major causes of cirrhosis and CLD are MASLD, viral hepatitis, and excessive or chronic alcohol intake. Other etiologies of CLD include autoimmune diseases including primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), alpha-1-antitrypsin deficiency, Wilson’s disease, and hemochromatosis.5 Table 1 highlights the causes of CLD and their association with kidney diseases.
| Liver diseases | Associated kidney disease |
|---|---|
| MASLD | CKD |
| Viral Hepatitis B and C | Glomerulonephritis (membranous nephropathy, IgA Nephropathy, Cryoglobulinemia) |
| PSC | Distal RTA |
| PBC |
Minimal change disease membranous nephropathy, distal RTA. |
| Obstructive Liver Diseases | Bilirubin nephropathy |
| Cirrhosis | ATN, HRS, IgA nephropathy, Ascites-associated compartment syndrome and congestive nephropathy, Cirrhotic cardiomyopathy–induced CRS. |
MASLD: Metabolic dysfunction–associated steatotic liver disease, CKD: Chronic kidney disease, IgA: Immunoglobulin A, RTA: Renal tubular acidosis, PSC: Primary sclerosing cholangitis, PBC: Primary biliary cholangitis ATN: Acute tubular necrosis, HRS: Hepatorenal syndrome, CRS: Cardiorenal syndrome.
CLD is known to significantly increase the risk of developing AKI and chronic kidney disease (CKD). In fact, AKI is a commonly identified complication of liver dysfunction, with a reported incidence ranging from 20 to 50 % in hospitalized patients.6,7 AKI in CLD can result from a multitude of causes, including volume depletion, bacterial infections such as spontaneous peritonitis, hypotension, shock, gastrointestinal bleeding, cardiac dysfunction, congestive nephropathy, abdominal compartment syndrome, glomerulonephritis, medications, and hepatorenal syndrome (HRS).8
AKI in liver diseases is associated with high morbidity and mortality.9 Liver transplantation (LT) has emerged as the mainstay of treatment for advanced liver disease, with simultaneous liver and kidney transplantation reserved for patients with ESKD or prolonged kidney disease before transplantation.10 AKI after LT is associated with a worse prognosis.11 This review will discuss the epidemiology, burden, clinical presentations, pathophysiology, prognosis, and management of AKI in CLD.
Epidemiology
The incidence of AKI in liver diseases varies widely depending on the types of liver diseases and the underlying etiology of kidney dysfunction, with prerenal causes accounting for 60–70% of all causes of AKI.12 An estimated 9000–35,000 patients with CLD in the United States experience HRS-AKI each year.13 According to some studies, approximately 22–50% of hospitalized patients with cirrhosis develop AKI.6,7
The burden of AKI in liver diseases is substantial, with some studies reporting a higher mortality rate, prolonged hospitalization, and increased healthcare costs.6 In a meta-analysis of 32 observational studies, AKI was associated with higher short- and long-term mortality rates.14 Ning Y. et al. reported a sixfold increase in in-hospital mortality rate among patients with cirrhosis and AKI compared to those without AKI.14 A worsening severity of AKI correlated with a higher rate of decompensation of cirrhosis and mortality.9 AKI is also a very common complication after LT, with a resultant higher mortality and poor survival of the transplanted liver, and a 30-day mortality and 1-year mortality rate of 16.5% and 31.1%, respectively.15 A recent retrospective study conducted in 2023 across 11 US hospitals revealed that prerenal AKI or hypovolemia accounted for 44% of all causes of AKI, with ATN and HRS accounting for 30% and 12%, respectively.16
Furthermore, studies have shown a correlation between MASLD and CKD.17 The risk of developing CKD is twofold higher in patients with MASLD compared to the general population.18 With the rising prevalence of MASLD-related cirrhosis leading to LT, studies have shown that more than 35% of cirrhosis related to MASLD is complicated by CKD stages 3–4 within 2 years of LT.19
Physiology of interplay between the liver and kidney
The interplay between the liver and kidney is complex, as both organs are closely interconnected and work to maintain the body’s homeostasis and metabolism. The liver and kidneys are vital organs that generate and excrete various toxins. Approximately 25% of circulatory blood volume passes through the liver, of which 75% traverses the portal venous system from the visceral organs, including the stomach, intestine, spleen, pancreas, and visceral fat.20
In a physiologic state, the consistency of hepatic blood flow is essential in maintaining the hemostatic role of the liver. Liver dysfunction can have a significant impact on the function of the kidney and vice versa. Portal hypertension is a complication of liver cirrhosis that arises from an increase in intrahepatic resistance as a consequence of liver architecture distortion due to fibrosis, nodularity, and increased intrahepatic vascular resistance.21 This is associated with the release of vasodilatory substances such as nitric oxide with resultant splanchnic and systemic vasodilation.22,23 In addition, bacterial translocation from the gastrointestinal tract, sepsis, and spontaneous peritonitis all result in splanchnic and systemic vasodilation, which ultimately results in a decrease in effective arterial blood volume and activation of the neurohumoral systems, including the renin-angiotensin-aldosterone system (RAAS).21 AKI develops as a consequence of the activation of the neurohumoral and arginine-vasopressin systems, including the RAAS which causes kidney arterial vasoconstriction and a reduction in kidney blood flow. Kidney hypoperfusion further activates the RAAS, leading to downstream production of angiotensin II, further increasing vasoconstriction. Ultimately AKI ensues, as well as hepatic stellate proliferation and fibrosis, further augmenting this vicious cycle. Figure 1 highlights the interplay between liver disease and AKI.
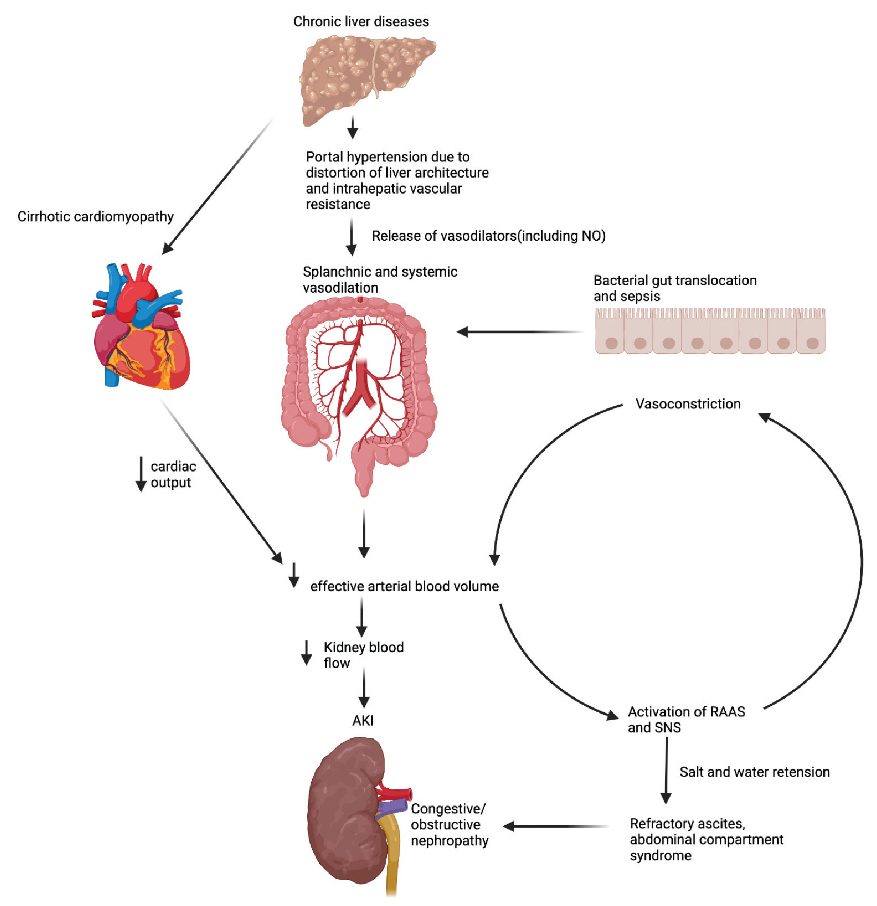
- Pathophysiological interplay between liver and kidney disease. Created with biorender.com. NO: Nitric oxide, RAAS: Renin-angiotensin-aldosterone system, SNS: Sympathetic nervous system, AKI: Acute kidney injury.
Causes of AKI in liver diseases
As mentioned previously, the liver and kidney are closely connected. The etiology of AKI in CLD is typically classified into prerenal, intrarenal, or post-renal causes.24
Prerenal causes
Prerenal causes of AKI include volume depletion, hypotension, or medications affecting glomerular hemodynamics such as diuretics. The general mechanism of prerenal AKI is diminished glomerular filtration due to decrease in kidney blood flow. HRS is a serious condition characterized by kidney failure due to liver dysfunction. The pathogenesis of HRS is complex involving splanchnic vasodilation due to the increased intrahepatic resistance causing portal hypertension, which is caused by nitric oxide, prostacyclin, and carbon monoxide, leading to decreased effective circulatory volume, activation of vasoconstrictive renin and angiotensin, and ultimately reduced kidney blood flow.23,25 Cardiomyopathy in cirrhosis can occur given increased systemic inflammatory cytokines due to sustained portal hypertension and portosystemic shunting, causing decreased cardiac output, increased right atrial pressure, and kidney tissue hypoxia.26 Kidney hypoperfusion can also result from medications, especially diuretics in patients with decompensated liver disease and ascites. Other causes of decreased perfusion to the kidneys include aggressive paracentesis, which causes fluid shifts and hypovolemia, and diarrhea, which can be caused by infection and lactulose.8,27 Abdominal compartment syndrome can also be a complication in patients with decompensated liver disease and ascites, leading to decreased kidney perfusion once intra-abdominal pressures are sustained above 20 mm Hg.28 In addition, the increased intra-abdominal pressure can lead to venous resistance due to renal vein compression and renal artery vasoconstriction induced by activation of RAAS and sympathetic nervous system leading to decreased glomerular perfusion and urine output.29,30
Intrinsic renal causes
Patients with liver dysfunction can also have intrinsic renal causes of AKI. Acute tubular necrosis (ATN) can develop due to prolonged prerenal AKI, hemorrhagic shock in the setting of variceal bleeding, spontaneous bacterial peritonitis (SBP), septic shock, or medications. SBP and sepsis are frequent etiological components of AKI, which set off a complex inflammatory response and cause extensive endothelial damage and microthrombi formation.31 This ultimately leads to the compromise of kidney perfusion and cellular damage in the kidney. Medications such as proton pump inhibitors and antibiotics including penicillin can also cause acute tubulo-interstitial nephritis.12,27 Glomerulonephritis can also occur in patients with CLD. IgA nephropathy has been frequently reported in patients with liver cirrhosis, presumably due to reduced clearance of immune complexes by the Kupfer cells of the liver.8,32,33 Patients with viral hepatitis, such as the Hepatitis B and C virus (HBV, HCV), can also develop membranous nephropathy and cryoglobulinemia, leading to decreased kidney function.8,33,34
Post-renal causes
Common causes of post-renal AKI include prostate hypertrophy, urothelial malignancies, nephrolithiasis, abdominal compartment syndrome due to ascites, SBP, and midodrine-induced obstructive uropathy leading to vasoconstriction and reduced kidney perfusion.35
Diagnosis
The diagnosis of AKI in CLD begins with a thorough physical examination including assessing vital signs, and laboratory and radiological investigations. Physical examination may reveal signs of volume depletion such as dry tongue, delayed capillary refill, reduced skin turgor, tachycardia, and hypotension. Laboratory investigations including a complete blood count could reveal profound anemia or thrombocytopenia suggestive of bleeding or hemorrhage. A kidney function panel is essential, since it may reveal metabolic disarrays including sodium, potassium, calcium, and phosphorus imbalances. Hypoalbuminemia could signify reduced protein synthesis by the liver or increased urinary protein losses, which raises suspicion for glomerular diseases. Urine studies, including a complete urinalysis, urine microscopic sediment examination, and urine sodium level, are essential for diagnosing the etiology of kidney injury. For example, urine sediment microscopy in ATN commonly shows muddy brown granular casts and urine sodium > 40 mEq/L, while urine sodium less than 20 mEq/L can be indicative of pre-renal AKI. Urine biomarkers can also be a useful tool in identifying the site of kidney injury. Common biomarkers to evaluate injury in the tubules include neutrophil gelatinase-associated lipocalin (NGAL),36 which is increased in HRS and ATN as well as kidney-injury molecule 1 (KIM-1),37 and liver-type fatty acid–binding protein (FABP).38 In addition, microalbumin which is a common marker for glomerular injury is markedly increased in ATN and glomerulonephritis. If glomerulonephritis is suspected, further workup with viral and immunological serologies is indicated, including antinuclear antibodies, C3, C4, hepatitis panel, HIV, and cryoglobulins. A urine analysis is key to differentiating glomerular from nonglomerular diseases. Red blood cells and proteinuria raise suspicion for glomerulonephritis, while bland urine sediment could signify volume depletion, cardiorenal syndrome (CRS), or HRS, which is a diagnosis of exclusion.39 Measuring intra-abdominal pressure could diagnose intra-abdominal hypertension or abdominal compartment syndrome. Imaging with ultrasonography and echocardiogram can identify obstructive uropathy and CRS as causes for AKI, respectively. Ultimately, a kidney biopsy could be indicated for a definitive diagnosis.
The diagnosis of HRS-AKI or CKD is challenging due to nonspecific clinical presentation and is often a diagnosis of exclusion. HRS prerequisites include decompensated cirrhosis, absence of shock and nephrotoxic medication use, no response to volume expansion and diuretic withdrawal, as well as the absence of parenchymal disease defined as proteinuria > 500 mg/d or hematuria <50 red blood cells (RBCs) per HPF. Over the years, the International Club for Ascites (ICA) has formulated criteria for the diagnosis of HRS [Table 2].8
| HRS-AKI (previously HRS Type 1) | HRS-CKD (previously HRS-type2) | |
|---|---|---|
| Definition | Increase in serum creatinine by 0.3 mg/dl in <48 hrs + UOP decrease ≤ 0.5 mL/kg body weight for ≥ 6 hours or increased serum creatinine by 50% from stable baseline within 3 months | Estimated GFR <60 mL/min per 1.73m2 for ≥ 3 months in the absence of other structural causes of kidney failure. |
| Additional criteria |
Presence of cirrhosis Absence of shock or sepsis No recent or current use of nephrotoxic drugs Absence of structural parenchymal diseases evidenced by proteinuria >500 mg/day, hematuria >50 RBC per HPF No abnormal ultrasound findings No evidence of kidney hypoperfusion indicated by FENa <0.1% No response (either full or partial) after at least 24 h of diuretic withdrawal, volume expansion with albumin 1 g/kg of body weight per day up to a maximum of 100 g/day. |
|
HRS-AKI: Hepatorenal syndrome-acute kidney injury, GFR: Glomerular filtration rate, HPF: High power field, FENa: Fractional excretion of sodium, UOP: Urine output, CKD: Chronic kidney disease, HRS-CKD: Hepatorenal syndrome-chronic kidney disease.
Management
General management
Treatment of kidney dysfunction in patients with CLD varies according to etiology [Table 3]. The management involves several strategies aimed at addressing the underlying cause while supporting kidney function. It is therefore important to identify the underlying causes of AKI in CKD which include hypovolemia, HRS, drug-induced kidney injury, infections, and sepsis.
| Supporting evidence | Management | |
|---|---|---|
|
Prerenal Decrease effective arterial volume due to GI bleed, iatrogenic diarrhea from laxatives and diuretics. |
Hypotension and signs of volume depletion, Urine sodium <20 mmol/l, improvement in AKI with volume expansion with albumin | Stop diuretics/laxatives. Volume expansion with albumin |
| Cirrhotic cardiomyopathy (CRS) | Elevated JVD, peripheral edema, reduced EF on echocardiogram, urine sodium <20mmol/l | Diuresis + heart failure GDMT |
| Hepatorenal syndrome | ICA criteria [Table 2] |
Vasoconstrictor therapy: terlipressin, octreotide Liver transplantation if refractory |
| Intrinsic renal | History of hypotension, gastrointestinal bleeding, or prolonged prerenal event Urine sodium >40 mmol/l Urinalysis and urine sediment examination: ATN: renal tubular epithelial cells + muddy brown/granular casts, ATIN: WBC + WBC casts Glomerulonephritis: Dysmorphic RBCs + RBC casts |
ATN: Expectant management. KRT if indicated ATIN: Stop offending agent. Steroids if required Glomerulonephritis: Treatment of associated viral infection Immunosuppression if required |
| Ascites-associated abdominal compartment syndrome | Increased intra-abdominal pressure >20 mmHg. | Large volume paracentesis with albumin |
| Post-renal/Obstructive Uropathy | Hydronephrosis on kidney ultrasound. | Consult urology. Treatment of the cause. |
GI: Gastrointestinal, ATN: Acute tubular necrosis. ATIN: Acute tubulointerstitial nephritis, CRS: Cardiorenal syndrome, JVD: Jugular venous distension, GDMT: Goal-directed medical therapy, ICA: International Club of Ascites, EF: Ejection fraction, KRT: Kidney replacement therapy, AKI: Acute kidney injury.
Volume expansion and vasoconstrictor therapy
In suspected HRS, initial plasma volume expansion with albumin for two consecutive days (1 g/kg of body weight, maximum 100 g/day) can help differentiate between prerenal AKI and HRS, with improvement in kidney function in prerenal AKI.40 HRS treatment includes vasoconstrictors such as terlipressin, norepinephrine, or midodrine plus octreotide with a combination of albumin.41 Table 4 shows different treatment options for HRS. Vasoconstrictors are commonly used for the management of HRS-AKI and terlipressin is widely used in Europe. A phase 3 clinical trial (CONFIRM trial) was conducted to confirm the effectiveness and safety of terlipressin in addition to albumin in the management of HRS-AKI. The trial showed that terlipressin was effective in HRS reversal compared to placebo while decreasing the need for kidney replacement therapy (KRT). However, terlipressin was associated with significant side effects including respiratory failure.42 The mechanism of action of terlipressin involves vasoconstriction of smooth muscles leading to splanchnic vasoconstriction.43 Terlipressin is now approved by the Food and Drug Administration (FDA) for the management of HRS-AKI in the United States.44 Prior studies have shown improvement in kidney function with vasoconstrictors, with a target increase in Mean Arterial Pressure (MAP) by >15 mmHg.45,46 Albumin has been shown to increase the effectiveness of terlipressin when used together.47 As a plasma expander, albumin helps improve the intravascular volume, counteracting the vasodilatation associated with liver cirrhosis. This volume expansion contributes to enhance kidney perfusion, mitigating kidney vasocontraction and hence counteracting HRS. Overall, the combination of albumin and terlipressin is a key therapeutic strategy for HRS, addressing both circulatory dysfunction and kidney failure.
| Medication | Mechanism | Comments |
|---|---|---|
| Terlipressin plus albumin | Synthetic vasopressin analogue, V1>>V2 receptor agonist resulting in splanchnic and systemic vasoconstriction. Reduces portal blood flow and increases effective arterial volume and MAP with increase in blood flow to the kidney |
Administered as a continuous infusion or IV bolus slowly over 2 min via peripheral or central line every 6 h with albumin concurrently. Discontinue after 14 days if sCr ≥ baseline Side effects include respiratory failure, hyponatremia, or ischemic events including mesenteric ischemia, acute MI, peripheral gangrene |
| Norepinephrine plus albumin | Alpha-1 and beta-1 adrenergic receptor agonist causing vasoconstriction and increased cardiac contractility | Use in ICU setting. |
| Midodrine, octreotide, and albumin | Midodrine is a selective alpha-1 adrenergic agonist leading systemic vasoconstriction; inhibits endogenous vasodilators. When combined with octreotide and albumin, it improves arterial blood volume and increases renal blood flow |
Used if terlipressin is unavailable. Midodrine or octreotide is not used as monotherapy |
V1: Vasopressin receptor 1, V2: Vasopressin receptor 2, MI: Myocardial ischemia, sCr: Serum creatinine, ICU: Intensive care unit, MAP: Mean arterial pressure.
Kidney replacement therapy
Historically, KRT has been used in the management of AKI in cirrhosis. In advance liver disease with HRS, KRT is commonly used as a bridge to liver transplant.43 There are various options for KRT including intermittent hemodialysis, slow sustained low efficiency dialysis (SLED), and continuous kidney replacement therapy (CKRT). The choice between IHD and CKRT should be based on hemodynamic factors and CKRT is a preferred option for dialysis for patients with hemodynamic instability and risk of cerebral edema.48 In centers where CKRT is unavailable due to high cost or unavailability of experts, SLED is a safe option as it offers similar hemodynamic stability as CKRT at a reduced cost in the management of HRS.49 Intermittent hemodialysis is generally not tolerated in patients with hemodynamic instability due to risk of further worsening hypotension leading to circulatory shock.
Complications of KRT include bleeding and infections with central venous access placement, and intradialytic hypotension complicated by hemodynamic instability in HRS.50 Splanchnic vasodilation resulting in decreased circulating volume and low MAP as well as hemodynamic instability caused by rapid fluid and solute shifts during dialysis can lead to hypotension and cerebral edema.43
Liver transplantation
LT is the optimal treatment for patients with HRS-AKI since it corrects the underlying liver failure and can cure HRS-AKI. The Model for End-Stage Liver Disease (MELD) scoring system is generally used to prioritize patients for liver allocation, based on factors like serum creatinine (sCr), international normalized ratio (INR), sodium level, and bilirubin. Simultaneous Liver and Kidney Transplantation (SLKT) in HRS-AKI patients is controversial, with updated criteria for SKLT lacking consistency in long-term benefits compared to LT alone.46,51
Prognosis and outcomes
Although the prognosis of AKI in liver diseases is generally poor, it varies depending on the severity of the underlying liver disease and the etiology of AKI.52,53 HRS is associated with an overall survival rate of 50% at 1 month and 20% at 6 months.52 The mortality rate of HRS-AKI is nearly 100% without LT. Although terlipressin has been associated with a higher rate of HRS reversal, it does not offer mortality benefits and is associated with respiratory failure.54 AKI in liver diseases is associated with progression to CKD and end-stage kidney diseases (ESKD) even after LT.
AKI in CLD affects outcomes of LT. Studies have shown that pre-transplant AKI is associated with higher post-LT morbidity and mortality.55,56 The incidence of AKI post-LT varies widely from 17% to 94%57 with up to 7.7% requiring KRT.15 The impact of post-LT AKI is substantial, with an increased prolonged hospital stay, utilization of resources, and healthcare cost. A pooled analysis of 38 cohort studies showed a 30-day mortality rate of 16.5% and a 1-year mortality rate of 31% among post-LT patients who developed AKI after transplantation.15 In addition, liver graft failure is significantly higher in patients with post-LT AKI compared to those without post-LT AKI.15
AKI is a serious complication of CLD associated with increased morbidity and mortality. Early diagnosis and management of AKI in CLD is imperative for survival. Terlipressin has emerged as a promising therapy for the reversal of HRS-AKI without improvement in mortality. More research is required to identify novel therapies to improve outcomes of AKI in patients with CLD.
Conflicts of interest
There are no conflicts of interest.
References
- The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global burden of liver disease: 2023 Update. J Hepatol. 2023;79:516-37.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of death among patients with hepatocellular carcinoma according to chronic liver disease etiology. Cancers (Basel). 2023;15
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global epidemiology of chronic liver disease. Clin Liver Dis (Hoboken). 2021;17:365-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury development and impact on clinical and economic outcomes in patients with cirrhosis: An observational cohort study over a 10-year period. Eur J Gastroenterol Hepatol. 2023;35:497-504.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? In: Kidney International. Vol 92. Elsevier B.V.; 2017. p. :1058-70.
- [Google Scholar]
- Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5:127-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Simultaneous liver kidney (SLK) allocation policy [Internet]. Available from: http://optn.transplant.hrsa.gov/governance/policies/
- Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009;15:475-83.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatorenal syndrome in hospitalized patients with chronic liver disease: Results from the Nationwide Inpatient Sample 2002-2012. J Investig Med. 2016;64:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of acute kidney injury on the risk of mortality in patients with cirrhosis: A systematic review and meta-analysis. Ren Fail. 2022;44:1-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence and impact of acute kidney injury after liver transplantation: A meta-analysis. J Clin Med. 2019;8
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence and outcomes of acute kidney injury including hepatorenal syndrome in hospitalized patients with cirrhosis in the US. J Hepatol. 2023;79:1408-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014;11:e1001680.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Non-Alcoholic Fatty Liver Disease: The Emerging Burden in Cardiometabolic and Renal Diseases. Diabetes Metab J. 2017;41:430-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. Journal of the American Society of Nephrology. 2005;16:180-8.
- [CrossRef] [PubMed] [Google Scholar]
- Regulatory processes interacting to maintain hepatic blood flow constancy: Vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res. 2007;37:891-903.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in patients with cirrhosis. New England Journal of Medicine. 2023;388:733-45.
- [CrossRef] [PubMed] [Google Scholar]
- Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-41.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Kidney Injury: Pre-renal, Intra-renal and Post-renal. In 2020. p. 23–44.
- Role of prostacyclin in hemodynamic alterations in conscious rats with extrahepatic or intrahepatic portal hypertension. Hepatology. 1993;18:621-7.
- [PubMed] [Google Scholar]
- Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105-10.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in patients with liver disease. Clinical Journal of the American Society of Nephrology. 2022;17:1674-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Abdominal compartment syndrome. Crit Care. 2000;4:23-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of increased renal parenchymal pressure on renal function. J Trauma. 2000;48:874-7.
- [CrossRef] [PubMed] [Google Scholar]
- The renal hemodynamic and functional effects of external counterpressure. Surg Gynecol Obstet. 1972;134:253-8.
- [PubMed] [Google Scholar]
- Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int. 2009;29:415-9.
- [CrossRef] [PubMed] [Google Scholar]
- Strong association between IgA nephropathy and hepatitis B surface antigenemia in endemic areas. Clin Nephrol. 1988;29:229-34.
- [PubMed] [Google Scholar]
- Association of liver cirrhosis related IgA nephropathy with portal hypertension. World J Gastroenterol. 2007;13:5783.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical remission of IgA nephropathy in an HIV-positive patient after combined treatment with tonsillectomy and steroid pulse therapy. CEN Case Rep. 2015;4:157-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Midodrine: insidious development of urologic adverse effects in patients with spinal cord injury: A report of 2 cases. Adv Ther. 2007;24:712-20.
- [CrossRef] [PubMed] [Google Scholar]
- Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013;17:R29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237-44.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hepatorenal syndrome misdiagnosis may be reduced using inferior vena cava ultrasound to assess intravascular volume and guide management. Ren Fail. 2023;45:2185468.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-74.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety profile of albumin administration for patients with cirrhosis at high risk of hepatorenal syndrome is dose dependent. Gastroenterol Rep (Oxf). 2015;3:216-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. New England Journal of Medicine. 2021;384:818-28.
- [CrossRef] [PubMed] [Google Scholar]
- The current management of hepatorenal syndrome-acute kidney injury in the United States and the potential of terlipressin. Liver Transpl. 2021;27:1191-202.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in patients with cirrhosis. J Clin Transl Hepatol. 2015;3:195-204.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hepatorenal acute kidney injury and the importance of raising mean arterial pressure. Nephron. 2015;131:191-201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current position of vasoconstrictor and albumin infusion for type 1 hepatorenal syndrome. World J Gastrointest Pharmacol Ther. 2015;6:28-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: Results of a prospective, nonrandomized study. Hepatology. 2002;36:941-8.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: A meta-analysis. Crit Care Med. 2008;36:610-7.
- [CrossRef] [PubMed] [Google Scholar]
- The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11:32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognosis of Acute kidney injury and hepatorenal syndrome in patients with cirrhosis: A prospective cohort study. Int J Nephrol. 2015;2015:108139.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of the etiology of acute kidney injury on outcomes following liver transplantation: Acute tubular necrosis versus hepatorenal syndrome. Liver Transplantation. 2012;18:539-48.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and short-term outcome of acute kidney injury in patients with acute-on-chronic liver failure: A meta-analysis. J Viral Hepat. 2020;27:810-7.
- [CrossRef] [PubMed] [Google Scholar]
- Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Me. 2021;384:818-28.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of survival after liver transplantation by pre-transplant parameters. Scand J Gastroenterol. 2008;43:736-46.
- [CrossRef] [PubMed] [Google Scholar]
- Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us? Am J Transplant. 2006;6:2651-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin Transplant. 2017;31:e12863.
- [CrossRef] [PubMed] [Google Scholar]







