Translate this page into:
Adrenocortical Suppression in Children with Nephrotic Syndrome Treated with Low-Dose Alternate Day Corticosteroids
Address for correspondence: Dr. M. Mantan, Department of Pediatrics, Maulana Azad Medical College, New Delhi - 110 002, India. E-mail: muktamantan@hotmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Corticosteroids form the mainstay of therapy for all forms of nephrotic syndrome. The long-term use of this medication is associated with serious side effects including adrenocortical suppression. The primary objective of this study was to identify adrenocortical suppression (assessed by single morning serum cortisol levels) in children with nephrotic syndrome on treatment with low-dose alternate day steroids. This cross-sectional study was conducted in the Department of Pediatrics in a tertiary care hospital from January 2014 to January 2015. Seventy children (1–18 years) with nephrotic syndrome (steroid sensitive and resistant) who were in remission and on low-dose alternate day steroids for at least 8 weeks or had received steroids of 2 mg/kg/d for at least 2 weeks in the last 1 year (infrequent relapsers) were enrolled. Relevant history was taken, clinical examination was done and blood samples were drawn for serum cortisol, lipid profile, kidney function tests, fasting blood sugar, glycated hemoglobin (HbA1c), and serum albumin. Forty percent (28/70) children had adrenocortical suppression as assessed by low morning serum cortisol levels. The mean serum cortisol levels were 188 nmol/L and were significantly lower in frequently relapsing individuals (85.9 nmol/L) as compared to other types of nephrotic syndrome (P = 0.05). The prevalence of adrenocortical suppression was higher in steroid-resistant patients (57%) as compared to 28% in frequently relapsing and 11% in steroid-dependent patients. Fifty-seven percent of patients with adrenocortical suppression had short stature while 50% had obesity. All individuals had normal serum HbA1c levels. The cumulative steroid doses and total duration of corticosteroid therapy were significantly higher in patients with adrenocortical suppression. Children with nephrotic syndrome treated with low-dose alternate day steroids have a high prevalence of adrenocortical suppression on screening with single morning cortisol sample. Those with frequently relapsing or steroid-resistant diseases are at a higher risk of suppression.
Keywords
Adrenocortical suppression
corticosteroids
cortisol levels
Introduction
Children with nephrotic syndrome are exposed to prolonged immunosuppressants. More than 90% children presenting with nephrotic syndrome in childhood respond to oral steroids. About 60% of these have a frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) that requires a prolonged period of steroid therapy or other immunosuppressants.[1] Patients with steroid-resistant disease (SRNS) are treated with calcineurin inhibitors, intravenous cyclophosphamide, intravenous steroids, purine antagonists such as mycophenolate mofetil and most recently rituximab. Most regimens involving these drugs also include administration of low-dose alternate-day prednisone or prednisolone.[2]
Adrenal suppression has been demonstrated after exposure to even 5 days of high-dose glucocorticoid therapy.[3] Longer-acting glucocorticoid formulations tend to be associated with a higher risk of adrenal suppression. While daily steroid therapy leads to adrenocortical suppression in a majority, low-dose alternate day steroids are presumed to be relatively safer.
The clinical presentation is different in acute and chronic adrenal insufficiency. Patients with acute adrenal insufficiency present with dehydration, hypotension, hypoglycemia, and altered mental status while those having chronic adrenal insufficiency present with fatigue, anorexia, loss of appetite, weight loss, myalgias, psychiatric symptoms, and abdominal pain.[456] Most of the symptoms of chronic insufficiency are rather nonspecific and need a high index of clinical suspicion for making a diagnosis.
The tests for the diagnosis of secondary adrenal suppression may be divided into provocative and nonprovocative tests. Provocative tests include high or low-dose cosyntropin stimulation and insulin tolerance tests. The nonprovocative tests include morning serum cortisol levels and overnight urinary free cortisol estimation.[678910]
The early morning serum cortisol (before 8:00 AM) levels are often used for screening of patients at risk of secondary adrenal suppression because of the difficulty, risk (in case of insulin-induced hypoglycemia), and cost of stimulation tests.[6711] Furthermore, it is an easy and practical test to perform in the developing countries. Different studies have used varying lower cutoffs of morning serum cortisol levels for biochemical evidence of adrenal suppression. An 8 AM cortisol level of ≤3 μg/dl (83 nmol/L) is suggestive of the diagnosis while a value ≥18 μg/dl (497 nmol/L) essentially eliminates it. A cutoff of 138 nmol/l (<5 μg/dl) is suggestive of adrenal suppression.[6]
A 4-week course of glucocorticoids, in patients being treated for leukemia, resulted in adrenal suppression for 8 weeks after discontinuation of therapy. The duration of corticosteroid therapy was positively correlated with the duration of adrenal suppression.[12] A study conducted by Wilson et al. in patients receiving oral prednisolone for asthma found that none of the patients receiving 5 mg prednisolone daily for 4 days had decreased plasma cortisol levels, but when the dose was increased to 10 and 20 mg, there was a significant decrease in serum cortisol levels even after 4 days of therapy.[13] A recent Cochrane review that looked into adrenocortical suppression caused by corticosteroid therapy in patients with acute lymphoblastic leukemia concluded that the adrenal cortex can stay suppressed even after the cessation of daily steroids for up to 12 months. Most of these patients were on long-acting corticosteroids which included dexamethasone and prednisolone.[14] A study conducted by Hansen et al. in 1982 on children with idiopathic nephrotic syndrome treated with corticosteroids showed 22 out of the 42 (52.5%) children enrolled for the study had adrenal suppression as assessed by low morning serum cortisol.[15] Another study enrolled 32 children with nephrotic syndrome on treatment with low-dose alternate-day corticosteroid therapy for over 12 months. Following low-dose adrenocorticotropic hormone (ACTH) stimulation, 62.5% individuals were found to have adrenal suppression.[16]
While multiple studies for adrenocortical suppression in children with corticosteroid therapy have been carried out in conditions such as acute lymphoblastic leukemia, chronic asthma, juvenile rheumatoid arthritis, there is a paucity of literature in nephrotic syndrome.[141718192021] With the increasing prolonged use of corticosteroids in the childhood nephrotic syndrome, there is a need to look into the adverse effects of such therapy. The aim of this study was to identify whether children exposed to low doses of alternate day steroids for treatment of nephrotic syndrome are at a risk of adrenocortical suppression.
Methods
This cross-sectional study was conducted in the Department of Pediatrics of a tertiary care teaching hospital between January 2014 and January 2015. The individuals of the study were children who visited the outpatient department or pediatric nephrology services at the hospital, fulfilled the inclusion criteria and gave consent for the study. The study protocol was approved by Institute's ethical committee (F.11/IEC/MAMC/10/No. 199 dated 20/11/13).
Inclusion criteria
The study included children (1–18 years) with nephrotic syndrome in remission and who were receiving low-dose alternate-day corticosteroid therapy (<1 mg/kg/day) for at least 8 weeks before enrolment. Participants who had received daily corticosteroids for ≥2 weeks in the last 1 year and were presently not on steroids were also enrolled for comparison. Both steroid sensitive (infrequent relapsers, steroid dependent, and frequently relapsing course) and steroid-resistant patients (now in remission) were included in the study.
Exclusion criteria
The study excluded children with nephrotic syndrome who had received daily corticosteroid therapy in the last 8 weeks. In addition, children having serious bacterial infections such as pneumonia, cellulitis, sepsis, meningitis, or any reasons for hospitalization were excluded from the study.
Sample size
The sample size by estimating the proportion of children with adrenocortical suppression with likely population prevalence of 20%, confidence level of 95%, and precision of 10% was calculated to be 64. Taking into account blood sample wastage (during storage) of 10%, a sample size of 70 was used. Previous studies have shown the prevalence of adrenal suppression, in patients with different diseases on low-dose alternate day corticosteroids, to be varying from 9% to 80%.[13141516]
Outcome variables
Primary
Proportion of patients having adrenocortical suppression as defined by an early morning (before 8:00AM) serum cortisol level of <138 nmol/l (5 μg/dl) was taken to be the primary outcome variable.
Secondary
Clinical and biochemical manifestations of adrenocortical suppression were taken as the secondary outcome variables.
The parents of children with nephrotic syndrome fulfilling the inclusion criteria were approached for the inclusion of the child in the study, and informed consent/assent was obtained. A prestructured Proforma consisting of clinical details and history regarding the age of onset, type of nephrotic syndrome (steroid sensitive or resistant), cumulative doses of steroids in the last 1 year, the current treatment regimen was filled. Clinical features of corticosteroid toxicity were assessed through a detailed history and clinical examination for moon facies, nuchal hump, purple striae, stretch marks, and cataracts. The anthropometry including weight and height, body mass index (BMI) and blood pressures of the subject were recorded. The WHO growth charts were used for comparison of anthropometry.
Venous blood sample was collected from each patient (before 8 AM) under all aseptic precautions on a predecided day (48 h after the last steroid dose) after overnight fasting. Biochemical investigations including lipid profile, blood urea, serum creatinine, electrolytes (sodium, potassium, and calcium), fasting blood sugar and serum protein (albumin and globulins) and glycated hemoglobin (HbA1c) levels were done. The biochemical investigations were done on the same day using an auto-analyzer of Olympus™ AU 400, Chemistry Analyzer, USA.
The sera was separated and stored at −20°C until tested for serum cortisol levels. The cortisol levels of all the patients were quantitatively determined by the Electrochemiluminescence immunoassay using The Roche-Hitachi Cobas™ e411 electrochemiluminescence machine.
Statistical analysis
The data entry was done using Microsoft Excel spreadsheet and analyzed using descriptive statistics. Mean and standard deviation (SD) was calculated for baseline characteristics and biochemical parameters. Student t-test or Mann–Whitney U-test was applied for comparisons depending on the use of mean or median for quantitative variables. Chi-square or Fisher's exact test was used for categorical variables. Logistic regression was used to determine the predictability of clinical and biochemical factors for adrenocortical suppression. For all comparisons, 5% probability (P< 0.05) was considered statistically significant.
Results
Of the total 70 children (52 boys, 18 girls) enrolled in the study, 13 (19%) were below 6 years, and 57 (81%) were >6 years of age with median age of 8 (2.5–18) years. Most (90%) children had disease onset before the age of 6 years. Thirty-two (45%) of the 70 children enrolled for the study had steroid-sensitive nephrotic syndrome (SSNS) while 38 (55%) of the children had SRNS now in remission. Out of the total 32 children having SSNS, 6 (18.8%) children had a steroid-dependent disease, 8 (25%) children had a frequently relapsing disease while 18 (56.2%) children had the infrequently relapsing disease.
Twenty-three (33%) out of the 70 patients enrolled had clinical features of corticosteroid toxicity. All of these 23 patients had a cushingoid appearance with hirsutism and 6 patients also complained of myalgias and weakness. Only 1 patient had cataract and needed surgery for it. Twenty six percent of the total patients were on enalapril due to hypertension detected previously.
Of the 70 children enrolled for the study, 17 (24%) received only corticosteroid therapy while 39 (56%) children received calcineurin inhibitors along with corticosteroids. Twelve (17%) received alkylating agents, and 2 (3%) had received other agents such as rituximab and mycophenolate mofetil in the past.
Of the 70 children enrolled for the study, 28 (40%) of the patients had low (<138 nmol/l) morning serum cortisol levels. The mean cortisol levels of the study were 188.8 (133.2) nmol/L. The mean levels were highest for the infrequently relapsing group (266.8 nmol/L) and lowest for frequently relapsing patients (85.9 nmol/L) [Figure 1]. Twenty-three (82.1%) of the participants having low cortisol levels had clinical features of steroid toxicity like cushingoid facies and hirsutism.
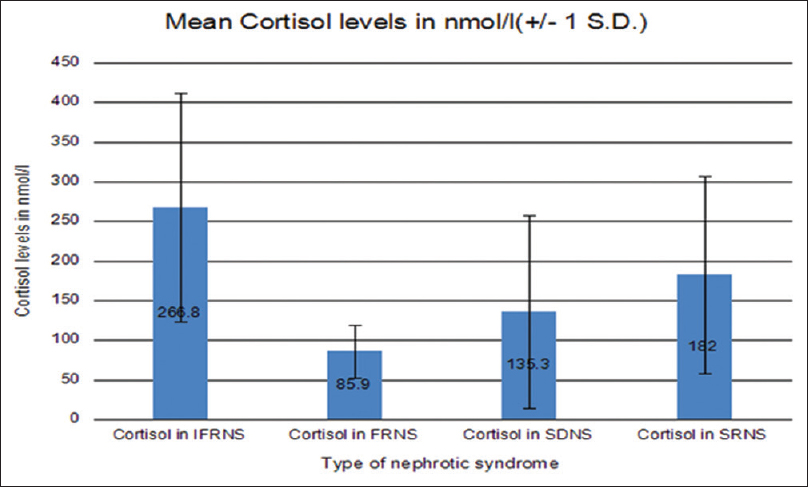
- Mean cortisol levels in different groups
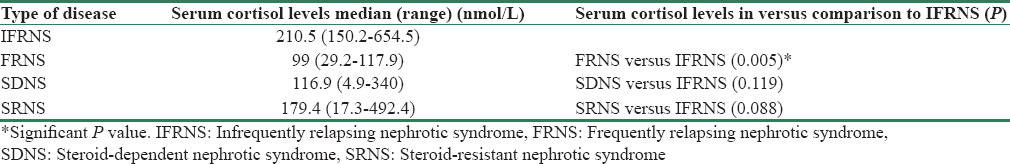
Of the 28 children with low serum cortisol levels, 57% had SRNS disease, 28% had frequently relapsing (FRNS), 11% had steroid dependent (SDNS), and only 4% had infrequently relapsing (IFRNS) course. Of these, 11/28 (39.3%) patients had serum cortisol levels below 50 nmol/L (very low levels). A comparison of serum cortisol levels in different subgroups of nephrotic syndrome showed that the levels were significantly lower in FRNS patients (P = 0.005) as compared to IFR while the difference did not reach statistically significant values in case of SDNS and SRNS participants [Table 1].
Of the 70 children, 14 (18.7%) had stopped alternate day steroids at least 3 months back. The mean cortisol levels were 209.9 nmol/L in this subgroup and only 1 patient had low cortisol levels. The mean cortisol levels of the remaining 56 on low doses of alternate day steroids were 179.1 nmol/L. While they were lower compared to IFR patients, they were not statistically significant (P = 0.52).
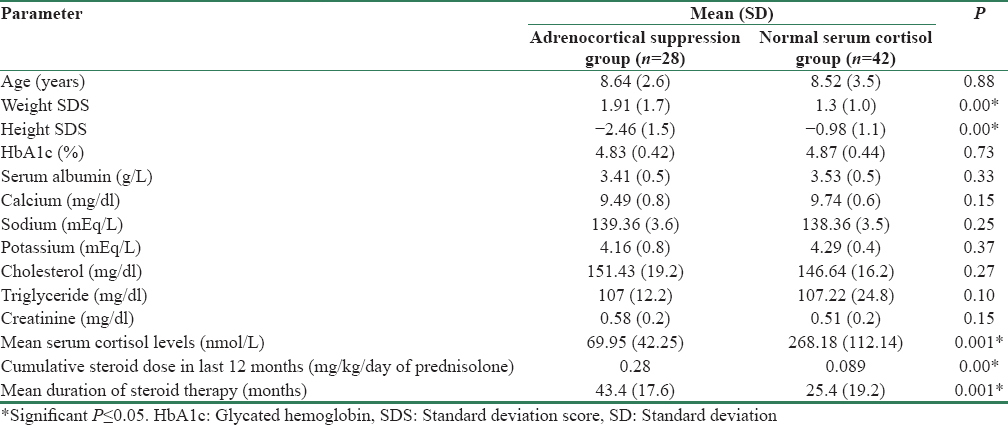
A comparison of the baseline characteristics of the participants with adrenal suppression and those without showed that children with adrenocortical suppression had higher weight standard deviation score (SDS) (1.91 vs. 1.31) (P = 0.00) and lower height SDS (−2.46 vs. −0.98) (P = 0.00) compared to those without suppression. In addition, the mean doses of prednisolone were significantly higher in the adrenocortical suppression group (0.28 mg/kg/d vs. 0.089 mg/kg/d) (P = 0.00). The mean duration of steroid therapy was longer in participants with adrenocortical suppression (P = 0.001). The mean serum cortisol levels in the suppressed group were 69.95 nmol/L as compared to 268.18 nmol/L in the nonsuppressed group (P = 0.001). Rest of the biochemical features were similar in both groups including HbA1c levels [Table 2].
Early morning serum cortisol levels had a negative correlation (Pearson correlation coefficient) with the BMI (r = −0.338), cumulative steroid dose over the last 12 months (r = −0.735), and the duration of steroid therapy (r = −0.342).
Discussion
Children with nephrotic syndrome are prone to adrenocortical suppression due to use of oral corticosteroids. Most patients with FRNS, SDNS, and SRNS course are administered low-dose alternate day steroids for prolonged periods with or without calcineurin inhibitors. While daily steroids can cause adrenocortical suppression for prolonged periods, low-dose alternate day steroids are considered to be a safe and less likely to cause suppression. Early identification of adrenocortical suppression may prevent life-threatening symptoms in children with adrenocortical suppression when they are exposed to any kind of severe stress such as infections or surgery.[56] This study was planned to identify adrenocortical suppression in individuals continued on low doses of alternate day steroids for prolonged periods for the treatment of nephrotic syndrome.
Of the 70 patients enrolled for the study, 52 (74%) of them were males with a male:female ratio of 2.9:1, which is similar to that observed in the past.[22] Nephrotic syndrome is more common in males than in females.
Most (55%) of the 70 children in this study had SRNS. This was predominately due to a referral bias. Among the 32 patients having SRNS disease, 58% had late steroid resistance. This proportion is similar to previously conducted studies in Asian children with SRNS.[2223] Twenty-three (33%) out of the enrolled 70 patients had clinical features of corticosteroid toxicity that included cushingoid features and hirsutism. Twenty-six percent patients had hypertension during the disease course. A previous study that included patients with SRNS participants showed that 34% of the patients had cushingoid facies and 27% the patients had hypertension at the time of enrolment.[23]
Of the 70 children included in the present study, 28 (40%) had serum cortisol levels below the cutoff of 138 nmol/L indicating adrenocortical suppression. Of these, 39.3% had very low serum cortisol levels (<50 nmol/L). Such low values are almost diagnostic of adrenal suppression.[611] In a previous study by Abeyagunawardena et al., 32 children on low-dose alternate day steroids for nephrotic syndrome underwent a low-dose ACTH stimulation test and 20 (62.5%) participants had adrenocortical suppression.[16] They chose a cutoff of 500 nmol/L for peak cortisol levels after the ACTH stimulation. Another study on children with idiopathic SSNS treated with corticosteroids showed 22 out of the 42 (52.5%) children had adrenocortical suppression as assessed by single morning cortisol levels.[15] This number further increased when they used a low-dose stimulation test. We chose the lower cutoffs for identifying adrenocortical suppression as we were doing only a single point assessment of levels for the purpose of screening.[17] Both the previous studies showed a higher prevalence of suppression as compared to the present study. However, these studies had enrolled a smaller number of subjects, which could have biased the results.
While stimulation tests are the gold standard for identification of adrenocortical suppression they are cumbersome and difficult to use in resource limited settings. A single morning cortisol helps in identifying at-risk patients who can be further be subjected to detailed evaluations including low-dose ACTH stimulation test.[679]
In a study by Smith et al. on children receiving inhaled steroids for asthma, 43 (20%) out of the 214 patients tested for serum cortisol levels had evidence of adrenocortical suppression. These 43 patients then underwent a low-dose ACTH stimulation test for confirmation of adrenocortical suppression and 20 (43.5%) had confirmed adrenocortical suppression on this test.[19]
Patients with FRNS course in our study had the lowest mean cortisol levels. They were possibly subjected to recurrent courses of steroids as compared to SRNS subjects who received cyclosporine early in the course of the disease. Drugs such as cyclophosphamide, levamisole, and mycophenolate mofetil are used before calcineurin inhibitors in subjects with FRNS disease and most regimens include low doses of prednisolone along with other agents for prolonged periods. The mean steroid doses over the past 12 months were 0.1 mg/kg/day in SDNS/SRNS group while they were 0.34 mg/kg/day in the FRNS group. The doses were at least 3 times higher in the FRNS group, and this appears to be the major reason for low cortisol levels.
On comparing the patients having normal adrenocortical function with those having suppression, no statistically significant difference was found in any of the biochemical parameters except serum cortisol levels, which were 268.18 versus 69.95 nmol/L. The mean steroid doses used in the adrenocortical suppression group were higher (0.28 mg/kg/d vs. 0.089 mg/kg/day) as compared to the nonsuppressed group. The mean steroid doses used in the previous study that looked into adrenocortical suppression were almost double (0.4 mg/kg/day) and therefore found a higher incidence of adrenocortical suppression (40% vs. 62.5%).[15] Furthermore, the duration of steroid use was higher in the suppressed group as compared to nonsuppressed ones (P = 0.001) in the present study. Patients in the suppressed group had lower mean height SDS scores compared to nonsuppressed group. This association has been reported earlier.[15] There was a negative correlation of low cortisol levels with doses and duration of steroid therapy. Furthermore, there was a negative correlation with BMI. This implies that prolonged courses of even low-dose steroid should be avoided and alternative agents should be used early for prevention of steroid toxicity.
While our study had more subjects with nephrotic syndrome in comparison to previous studies, the population chosen by us was more heterogeneous. This in fact was a major limitation as both steroid sensitive and steroid-resistant patients were included due to logistics. It is difficult to enroll subjects as per the sample size in single-center studies. This being a cross-sectional study, we decided to enroll all patients on low-dose alternate day steroids as the research question here was whether low-dose alternate day steroids causes suppression or not. More patients with steroid-resistant disease are likely to continue on low-dose steroids along with cyclosporine even after achieving complete remission. All subjects with steroid-resistant disease in the present study were in remission at the time of enrollment and were on low-dose alternate day corticosteroids with or without calcineurin inhibitors. Also, we used only single morning cortisol sample for assessment of suppression and did not follow it up with ACTH stimulation due to nonavailability. Studies with a more homogeneous population with adequate sample size and possibly the addition of stimulation test may answer the question of adrenocortical suppression better.
At present, there are no clear guidelines regarding the screening for adrenocortical suppression in children with nephrotic syndrome. While daily steroids cause suppression in most patients the use of low-dose alternate day therapy is traditionally thought to be relatively safe.
Based on the results of this study, we conclude that there is a high prevalence of adrenocortical suppression in children with nephrotic syndrome receiving even low doses of corticosteroids on alternate days. All subjects with nephrotic syndrome on treatment with low-dose alternate day steroids (below 0.5 mg/kg/d) for prolonged periods (>1 year) may be screened for adrenocortical suppression by single morning cortisol levels, and values below 138 nmol/l are suggestive of suppression. Very low values below 50 nmol/l can be diagnostic of suppression. A low-dose ACTH stimulation test may further be done to confirm the suppression depending on the availability of the investigation. However, as there is paucity of literature on this topic, further studies with a correlation of single morning doses with low-dose ACTH stimulation are needed for more definitive guidelines.
Financial support and sponsorship
None
Conflicts of interest
There are no conflicts of interest.
References
- Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev. 2016;10:CD003594.
- [Google Scholar]
- Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355:542-5.
- [Google Scholar]
- Management of complications of glucocorticoid therapy. Clin Chest Med. 1997;18:507-20.
- [Google Scholar]
- Corticosteroid-induced adverse events in adults: Frequency, screening and prevention. Drug Saf. 2007;30:861-81.
- [Google Scholar]
- Adrenal insufficiency: Still a cause of morbidity and death in childhood. Pediatrics. 2007;119:e484-94.
- [Google Scholar]
- A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30.
- [Google Scholar]
- Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83:2350-4.
- [Google Scholar]
- Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923-31.
- [Google Scholar]
- The one microgram adrenocorticotropin test in the assessment of hypothalamic-pituitary-adrenal function. Eur J Endocrinol. 1998;139:575-9.
- [Google Scholar]
- Time course of recovery of adrenal function in children treated for leukemia. J Pediatr. 2000;137:21-4.
- [Google Scholar]
- Systemic bioactivity profiles of oral prednisolone and nebulized budesonide in adult asthmatics. Chest. 1998;114:1022-7.
- [Google Scholar]
- Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database Syst Rev. 2015;8:CD008727.
- [Google Scholar]
- Adrenal responsiveness in children with steroid responsive idiopathic nephrotic syndrome. Int J Pediatr Nephrol. 1982;3:1-4.
- [Google Scholar]
- Adrenocortical suppression increases the risk of relapse in nephrotic syndrome. Arch Dis Child. 2007;92:585-8.
- [Google Scholar]
- Adrenal suppression: A practical guide to the screening and management of this under-recognized complication of inhaled corticosteroid therapy. Allergy Asthma Clin Immunol. 2011;7:13.
- [Google Scholar]
- Effects of long-term inhaled corticosteroids on adrenal function in patients with asthma. Ann Allergy Asthma Immunol. 2006;96:437-44.
- [Google Scholar]
- Prevalence of hypothalamic-pituitary-adrenal axis suppression in children treated for asthma with inhaled corticosteroid. Paediatr Child Health. 2012;17:e34-9.
- [Google Scholar]
- Moderate dose inhaled corticosteroid-induced symptomatic adrenal suppression: Case report and review of the literature. Clin Pediatr (Phila). 2012;51:1184-90.
- [Google Scholar]
- Effect of different corticosteroid regimens on hypothalamic-pituitary-adrenal axis and growth in juvenile chronic arthritis. J R Soc Med. 1983;76:452-7.
- [Google Scholar]
- Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid-resistant nephrotic syndrome. Kidney Int. 2012;82:1130-5.
- [Google Scholar]
- Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis. 2009;53:760-9.
- [Google Scholar]







