Translate this page into:
Allopurinol for prevention of progression of kidney disease with hyperuricemia
Address for correspondence: Dr. B. H. Santhosh Pai, S/o B. H. Narayan Pai, Sharada Nilaya, College Road, Bantwal, Mangalore - 574 211, Karnataka, India. E-mail: drbhspai22@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hyperuricemia is associated with hypertension and progressive chronic renal disease. This is a retrospective cohort study in chronic kidney disease (CKD) patients with hyperuricemia from 1998 to 2008. Patients were divided into two groups: treatment group who received allopurinol in a dose of 100 mg/day and the other group remained untreated. Clinical, hematologic, biochemical parameters and outcome were measured at baseline and 6 months, 1 year, and 2 years of treatment. A total of 183 patients were enrolled. Mean age of the allopurinol group was 50.15 ± 14.42 years and control group was 53.23 ± 13.86 years. Male-female ratios were 2.57:1 and 2.21:1 for the treatment and control groups, respectively. Baseline characteristics and the laboratory parameters were similar in both groups. Patients who received allopurinol had lower blood pressure at 6 months, 1 year, and 2 years when compared to baseline. There was a significant decrease in the serum uric acid (UA) levels in the treatment group at the end of 6 months, 1 year, and 2 years with respect to base line. An inverse correlation as noted between serum UA levels and the estimated glomerular filtration rate at 6 months, 1 year, and 2 years. Allopurinol treatment decreases blood UA levels and is associated with better blood pressure control and decreased progression of renal disease in CKD patients with hyperuricemia.
Keywords
Allopurinol
chronic kidney disease
uric acid
Introduction
Current research shows that serum uric acid (UA) levels are associated with hypertension,[12345] a risk factor for chronic kidney disease (CKD). A rat model of mild hyperuricemia demonstrated that mild elevations in UA, even within the normal limits, can cause hypertension and renal microvascular disease without causing urate crystal deposition in kidneys.[6] In a population-based study of Appalachian adults, increasing the serum UA levels were positively associated with CKD, independent of age, gender, smoking status, alcohol intake, education, diabetes mellitus, hypertension, body mass index (BMI), and total cholesterol.[7]
Recent epidemiologic data from 21,475 healthy volunteers who were followed prospectively for a median of 7 years suggest that elevated levels of UA independently increase the risk for new-onset kidney disease. Hyperuricemia could be a consequence of impaired kidney function, diuretic therapy or oxidative stress, such that elevated serum urate level represents a marker, rather than a cause of CKD. Strategies to reduce the serum UA, including dietary changes such as lower intake of fructose- and sugar-sweetened beverages and red meat[8] and UA lowering drugs like allopurinol[9] may be useful in preventing or arresting the progression of kidney disease. For animal models of established renal diseases, correction of the hyperuricemic state can significantly improve the blood pressure control, decreasing proteinuria and slowing the progression of renal disease.[10] Recent randomized controlled trials reported that UA-lowering medication with allopurinol was associated with a lower serum creatinine level in the treatment group compared to controls.[9] We therefore conducted a cohort study to analyze the renal effects of allopurinol treatment in CKD patients with hyperuricemic.
Materials and Methods
This study is a retrospective cohort analysis of CKD patients with hyperuricemia, attending out-patient department (OPD) of Nephrology at Nizam's Institute of Medical Sciences, Hyderabad from 1998 to 2008.
With a margin of error of 10 and confidence interval of 95% and 50% of response distribution the sample size was calculated to be 192. The details are shown in the Figure 1. The information about the patients was obtained directly from medical records preserved in the OPD of Nephrology. The records were periodically updated by doctors during each follow-up of the patient.
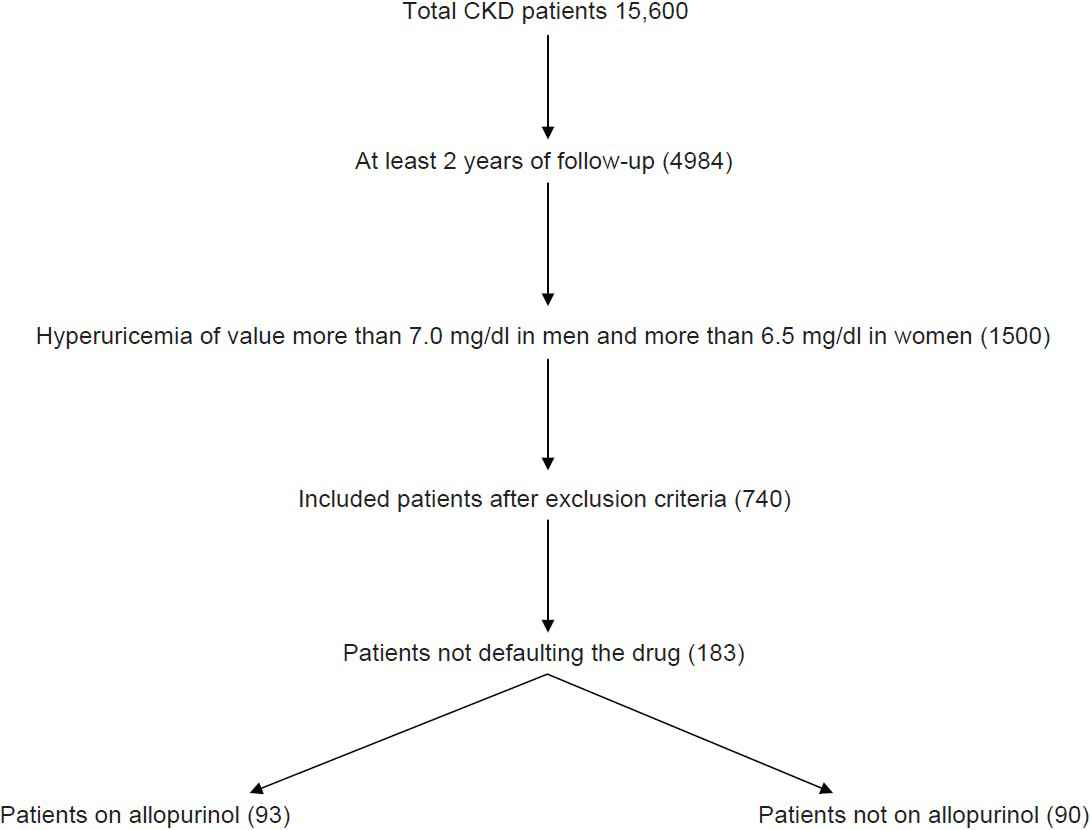
- Flow chart showing sample size collection
The included subjects had to fulfill the following inclusion criteria at the time of entry: estimated glomerular filtration rate (eGFR) lower than 90 ml/min, and serum UA > 7.0 mg/dl in men 6.5 mg/dl in women. Patients with allopurinol intolerance, active infections or inflammatory diseases, and chronic hepatopathy and those with a history of gouty arthritis and renal stones were excluded.
We collected data of CKD patients from January 1998 to December 2008. Data collected included gender, age, body weight, blood pressure, history of smoking and drinking alcohol, blood glucose, triglycerides, cholesterol, blood urea nitrogen, serum creatinine, serum UA concentration, 24 h urinary protein, liver function test, and the treatment received. Modification of the diet in the renal disease (MDRD) equation was used to calculate eGFR. We divided the patients into two groups: Treatment group who received allopurinol in a dose of 100 mg/day and the control group who did not receive allopurinol.
Subjects were followed-up at regular intervals for 2 years. During each follow-up session, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Laboratory parameters such as daily urinary protein excretion, hemoglobin level, white blood cell count, platelet count, serum creatinine level, alanine aminotransferase level, fasting total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, triglyceride level, and fasting UA level were all recorded.
The study end points were as follows:[1] stable renal function indicated by stable serum creatinine and stable eGFR level at the end of the study compared to base line[2] worsening of renal function indicated by an increase in serum creatinine level and/or decrease in eGFR compared to baseline, but not yet requiring dialysis.
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 11.0, April 2002 (SPSS Inc., Chicago, IL) Program for Windows XP. Values are expressed as mean ± standard deviation (SD). Categorical data were compared by means of Chi-square test, and continuous variables, by means of Student t-test. Comparison of various parameters between baseline and different intervals was performed by means of paired Student t-test. Statistical significance is defined as two-tailed P less than 0.05.
Results
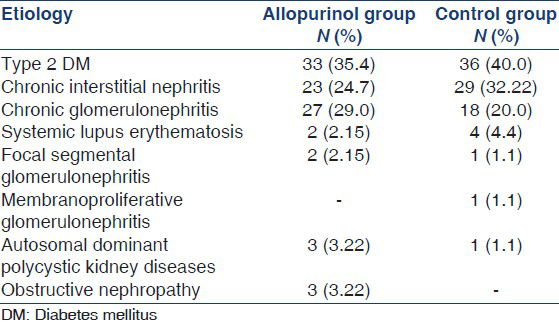
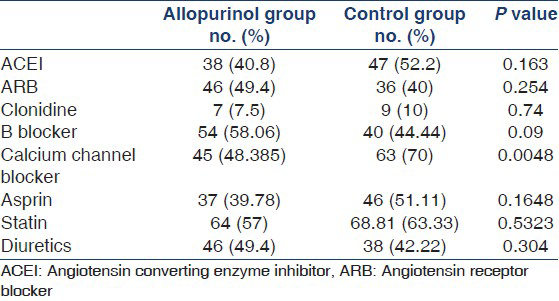
A total of 183 patients were enrolled in the study. Baseline characteristics and laboratory parameters are listed in Table 1. The most common cause of renal disease in both groups was diabetes mellitus. Other causes of renal disease in our study population are listed in Table 2. There was no significant difference in the medications used in both groups [Table 3] except for a higher percentage of patients in the control group used calcium channel blocker.

Blood pressure control
SBP and DBP of both groups at 6 months, 1 year, and 2 years are shown in Figure 2. When we compared the blood pressures between the two groups, there was a significant fall in the mean SBP and DBP at the end of 2 years in the allopurinol group when compare to the control. This may explain the higher percentage of patients in the control group used calcium channel blockers.
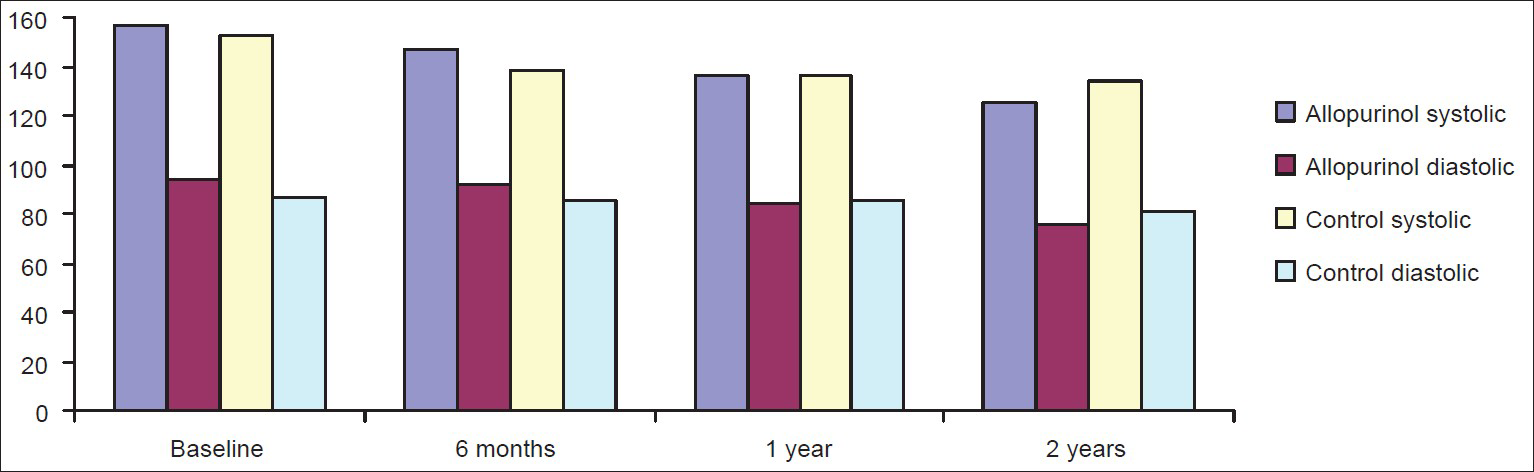
- Comparison of mean systolic blood pressure and diastolic blood pressure of chronic kidney disease patients of allopurinol and control group
Serum UA and proteinuria
Serum UA levels of all the subjects in allopurinol and control group at the start and end of the study are shown in Figure 3. Within the allopurinol group there was a significant fall in serum UA at 6 months, 1 year, and 2 years when compared to baseline.
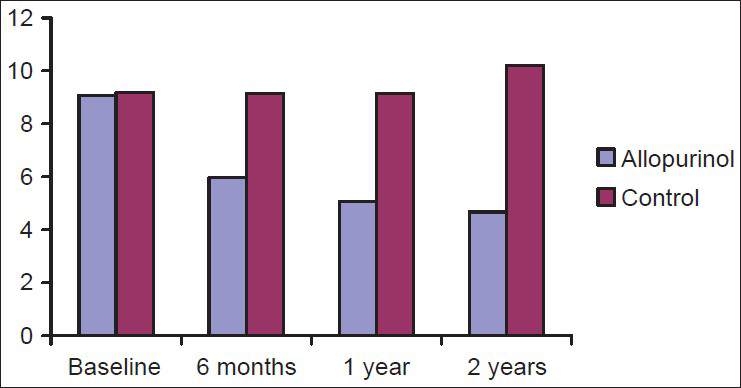
- Comparison of mean serum uric acid of chronic kidney disease patients in allopurinol and control group
There was no significant difference in the proteinuria [Figure 4] in allopurinol and control groups at baseline, 6 months and 1 year. At the end of 2 years, when compared to baseline, though there was a decrease in proteinuria in allopurinol group and increase in proteinuria in the control group, this was not statistically significant. However, control group had a significant increase in proteinuria compared to allopurinol group at the end of 2 years.
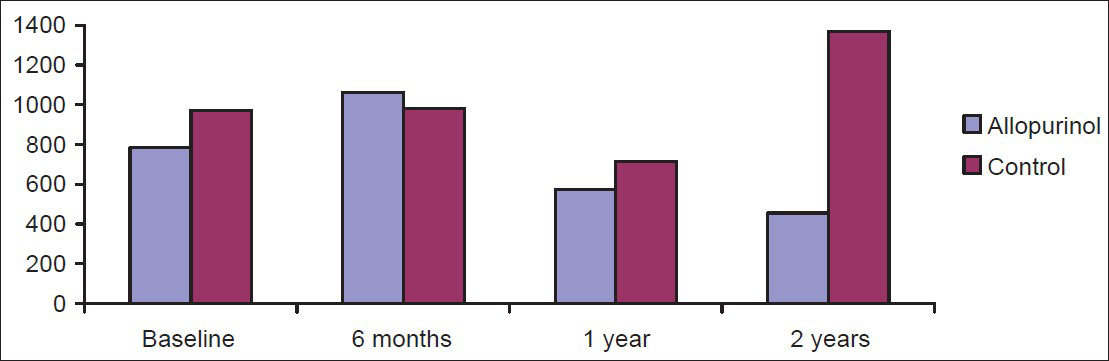
- Comparison of mean proteinuria chronic kidney disease patients in allopurinol and control group at 6 months, 1 year, and 2 years with the base line
Renal function
The eGFR of both the groups at 6 months, 1 year, and 2 years are shown in Table 4. eGFR was similar in both the groups at base line. In the control group, there was a significant fall in eGFR at 6 months, 1 year, and 2 years compared to baseline eGFR, whereas in allopurinol group there was no significant change in eGFR at 6 months, 1 year, and 2 years compared to baseline and there was a significant fall in eGFR at 1 year and 2 years in the control group when compared to allopurinol group.

Discussion
In patients with renal disease, there is decreased UA urinary excretion, and whether this will give rise to hyperuricemia depends on the gastrointestinal excretory compensation. Patients with CKD develop hyperuricemia as GFR declines; hence the prevalence of elevated serum UA in patients with CKD is higher.[11] In our study, the prevalence of hyperuricemia in CKD was 29.5%. In Siu et al. study[9] the prevalence of elevated serum UA levels in patients with renal diseases was 19.6%. It was an underestimation of the proportion of patients with co-existing hyperuricemia and renal disease as they excluded patients with advanced stages of renal disease.

In epidemiologic studies, urate levels were reported to be correlated with the development of chronic renal insufficiency in patients with hypertension and impaired renal function.[121314] In a recent community-based prospective study of 13,338 participants, UA level was described to be an independent risk factor for the development of kidney disease and mortality.[15] In addition, a prospective controlled trial by Sui et al.[9] examined the effect of decreasing UA level in patients with CKD and hyperuricemia. However, Fessel et al.[16] and Yu et al.[17] did not show a significant association between kidney dysfunction and hyperuricemia. A recent study by Michel et al.[18] also showed a weak association of hyperuricemia and progression of CKD though UA had a strong cross-sectional association with prevalent CKD.
Despite multiple epidemiological and prospective studies, the role of UA in the progression of kidney disease and development of kidney failure remains unclear.[1920] Hyperuricemia has been related to high blood pressure. This is thought to be mediated through activation of the renin-angiotensin system by multiple mechanisms; directly, by decreasing neuronal nitric oxide synthase in the juxtaglomerular apparatus,[21] indirectly, through decreasing renal perfusion by stimulating the afferent arteriolar vascular smooth cell proliferation[22] and through the induction of cyclo-oxygenase-2 in the macula densa and arterioles.[23] Our study showed 21.85% of patient with hyperuricemia had hypertension. It was reported that up to 50-70% of hyperuricemic patients had hypertension in other studies,[19] and conversely, 25% of hypertensive patients had elevated UA levels.[5] Hyperuricemia is a clinical finding in 25-40% of adult patients with untreated hypertension.[24]
In experimental rat models, controlling UA levels with allopurinol prevented the development of hypertension and renin and neuronal nitric oxide synthase level changes.[21] In our study, patients who received allopurinol had significantly lower mean SBP and DBP at 6 months (P < 0.0001), 1 year (P < 0.0001), and 2 years (P < 0.0001) when compared to baseline. Allopurinol by decreasing the serum UA levels decreases the blood pressure. This is in contrast to Siu et al.,[9] which showed that only SBP decreased in the treatment group after allopurinol treatment, but blood pressure was not significantly different from the control group. In Goicoechea et al.,[25] blood pressure control was similar in both groups, and no significant differences were observed in the follow-up period in SBP and DBP. The reason may be patients had established renal diseases with impaired renal function, and most patients had long-standing hypertension at the time of recruitment. Structural damage to the arteries and kidneys had been incurred, and the high blood pressure at that point probably was multifactorial in pathogenesis. Thus, although serum UA level was normalized, hypertension probably was irreversible. Therefore, it seems that to ameliorate the hypertensive effect of hyperuricemia, early treatment with allopurinol may be necessary, and once the disease has been established, the effect of decreasing serum UA levels may be limited.
Kang et al.[6] found that hyperuricemic rats showed greater proteinuria, greater blood pressure, and greater serum creatinine levels than controls, which were treated with allopurinol to decrease serum UA levels. However, Siu et al.,[9] could not show a benefit of using allopurinol in decreasing the amount of proteinuria in hyperuricemic patients. In our study, though there was mild increase in proteinuria in control group and mild decrease in proteinuria in allopurinol group compared to baseline, those were was not statistically significant. However, when we compared the levels of proteinuria at the end of 2 year, there was a significant increase proteinuria in the control group compared to allopurinol group. This suggests that allopurinol has definite beneficial effect in decreasing the proteinuria.
High UA level has been associated with a greater incidence of end-stage renal disease.[1926] The researchers found that mean eGFR decreased from 97 ml/min/1.73 m2 to 88 ml/min/1.73 m2 after a median of 59 months of follow-up, and that higher serum UA levels at baseline were associated with a greater risk of eGFR decline in 900 healthy normotensive adults. After adjusting for potential confounding factors (including BMI, blood glucose level, urinary albumin-to-creatinine ratio, baseline eGFR, age, and sex), the researchers found that each 59 μmol/l (1 mg/dl) increase in serum UA level at baseline was associated with a 23% increase in the risk of an eGFR decrease of >2 ml/min/year.[27] In a community based study among 13,338 individuals, the base-line serum UA level was associated with a significantly increased risk for developing kidney disease in univariate and multivariable analysis.[15]
In our study, control subjects showed a fall in the eGFR at 1 year and 2 years. Our results were similar to Siu et al.[9] and Goicoechea et al.[25] which also showed that allopurinol is able to slow the progression of renal diseases. Allopurinol was able to slow the progression of renal disease after a mean time of 23.4 ± 7.8 months.[25] The precise mechanism is not known, but probably related to multiple factors. UA has a number of detrimental effects. It can cause endothelial dysfunction, which can be improved with allopurinol,[28] and it can also activate circulating platelets[29] and impair endothelial nitric oxide production. In different small randomized controlled trials, allopurinol treatment resulted in the improvement of oxidative stress, endothelial function,[2930] and progression of CKD.[9] Hyperuricemia has also been shown to cause an increase in glomerular hydrostatic pressure, caused by direct UA stimulation of vascular smooth muscle cell proliferation in the afferent arterioles, which induces a more rigid vessel wall and loss of the autoregulatory and protective mechanisms. Arterial pressure then is transmitted directly to the glomerulus, causing glomerular hypertension, resulting in glomerular hypertrophy and sclerosis.[31] Allopurinol therefore, by diminishing serum UA levels, serve as an agent to decrease glomerular hydrostatic pressure indirectly and thus help alleviate renal damage.
As expected, there was a significant decrease in the mean serum UA levels in treatment group, and there was inverse correlation between serum UA levels and the eGFR at 6 months, 1 year, and 2 years. Goicoechea et al.,[25] also showed that there was a significant inverse correlation between UA levels and eGFR in the whole data and within each experimental group. This means that, the beneficial effect of allopurinol slowing down the progression of renal disease could be related to the decrease of UA level. Chonchol et al.[18] evaluated the association between hyperuricemia and progression of kidney disease in 5808 participants from the cardiovascular health system, demonstrating a 14% (odds ratio [OR] 1.14; Nearly, 95% confidence interval [CI] 1.04-1.24 per 1-mg/dl rise in UA) increase in kidney disease progression, defined by eGFR decline <3 ml/min/1.73 m2/year, but no relationship between baseline serum UA levels and incident CKD (OR 1.00; 95% CI 0.89-1.14) Kanbay et al.[32] reported that treatment of asymptomatic hyperuricemia improved renal function. Likewise, Siu et al.[9] reported that the treatment of asymptomatic hyperuricemia delayed progression of kidney disease. The results of our study are similar to other studies[925] in decreasing the progression of renal disease by allopurinol in CKD patients with hyperuricemia. The unique feature of our study is that, we had more number of patients and a longer follow-up time.
There are several limitations to our study. It was not designed in a randomized control, double-blinded fashion. The results of our study may be limited by the concomitant use of statins, antiplatelet and renin-angiotensin-aldosterone system blocker drugs. Although there were no baseline differences in the use of these drugs between the groups, these treatments could have been modified during the study according to good clinical practices and we could not delineate completely the possible beneficial effect contributed by these drugs preservation of kidney function.
We conclude that allopurinol might help in slowing down the progression of renal disease in CKD patients with hyperuricemia, but the mechanism is unclear.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28-33.
- [Google Scholar]
- The association between serum uric acid level and long-term incidence of hypertension: Population-based cohort study. J Hum Hypertens. 2006;20:937-45.
- [Google Scholar]
- Precursors of essential hypertension: Pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol. 1990;131:1017-27.
- [Google Scholar]
- Serum uric acid and hypertension: The olivetti heart study. J Hum Hypertens. 1994;8:677-81.
- [Google Scholar]
- A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888-97.
- [Google Scholar]
- The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrol Dial Transplant. 2010;25:3593-9.
- [Google Scholar]
- Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283-9.
- [Google Scholar]
- Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51-9.
- [Google Scholar]
- Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183-90.
- [Google Scholar]
- The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75(Suppl 5):S13-6.
- [Google Scholar]
- Regulation of renal urate excretion: A critical review. Am J Kidney Dis. 1998;32:917-33.
- [Google Scholar]
- Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol. 2000;27:1045-50.
- [Google Scholar]
- Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204-11.
- [Google Scholar]
- Impaired renal function gout: Its association with hypertensive vascular disease and intrinsic renal disease. Am J Med. 1982;72:95-100.
- [Google Scholar]
- Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239-47.
- [Google Scholar]
- Essential hypertension, progressive renal disease, and uric acid: A pathogenetic link? J Am Soc Nephrol. 2005;16:1909-19.
- [Google Scholar]
- Does asymptomatic hyperuricaemia contribute to the development of renal and cardiovascular disease? An old controversy renewed. Nephrology (Carlton). 2004;9:394-9.
- [Google Scholar]
- Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101-6.
- [Google Scholar]
- Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604-8.
- [Google Scholar]
- Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1-11.
- [Google Scholar]
- Uric acid as a marker for renal dysfunction in hypertensive women on diuretic and nondiuretic therapy. J Clin Hypertens (Greenwich). 2009;11:253-9.
- [Google Scholar]
- Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388-93.
- [Google Scholar]
- Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642-50.
- [Google Scholar]
- Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56:264-72.
- [Google Scholar]
- Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221-6.
- [Google Scholar]
- Blood coagulation and platelet economy in subjects with primary gout. Can Med Assoc J. 1963;89:1207-11.
- [Google Scholar]
- High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508-16.
- [Google Scholar]
- Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283:F1105-10.
- [Google Scholar]
- Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227-33.
- [Google Scholar]







