Translate this page into:
An Unusual Case of Relapsing and Remitting Acute Kidney Injury
Corresponding author: Lavanya S R Bhat, Department of Nephrology, Nizam’s Institute of Medical Sciences Punjagutta, Hyderabad, Telangana - 500 082, India. E-mail: lavanyasr2191@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhat L S R, Taduri G, Guditi S, Herur S, Bukka VC, Murugan P. An Unusual Case of Relapsing and Remitting Acute Kidney Injury. Indian J Nephrol. 2024;34:665-6. doi: 10.25259/ijn_437_23
Abstract
Paroxysmal nocturnal haemoglobinuria (PNH), although a rare type of acquired hemolytic anemia, can be life-threatening if not diagnosed early. Kidney involvement in PNH varies from reversible acute kidney injury to irreversible chronic damage. Here, we report a case of recurrent acute kidney injury in a young male requiring renal replacement support. Repeated history of AKI with coombs negative hemolytic anemia led us to perform PNH profile after ruling out other causes. Although kidney involvement in PNH is not apparent, this case shows the importance of having a high index of suspicion which will help in preventing further episodes of AKI and thus, chronic kidney disease burden.
Keywords
Acute kidney injury
Hemolytic anemia
Paroxysmal nocturnal hemoglobinuria
Chronic kidney disease
Hemosiderin deposits
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematological disease, caused due to acquired mutations that lead to the reduction or absence of glycosylphosphatidylinositol (GPI)-anchored proteins on the surface of blood cells.1 This disease is underdiagnosed as it masquerades as various clinical syndromes. Chronic hemolysis causing hemosiderosis in renal parenchyma will eventually lead to chronic kidney disease (CKD), one of the major determinants of prognosis. Although previous literature mentions 14% renal involvement in PNH patients, index renal presentation of the disease is very rare.
Case Report
A 29-year old man presented with a history of low-grade fever and dark-colored urine for 5 days. There was no history of cough, shortness of breath, or other urinary symptoms. On probing, the patient revealed history of similar episodes in the last 1 year. In his first episode, he had to undergo a few sessions of hemodialysis along with a blood transfusion. Even though the second and third episodes did not require hospital admissions, the patient had deranged kidney dysfunction with anemia; however, he never underwent detailed evaluation. On investigation, he was found to have Coombs-negative hemolytic anemia with renal dysfunction [Table 1]. The blood parameters showed the following values: hemoglobin- 7.4 g/dl, (PCV) Packed cell volume- 23.4 vol%, platelet count- 1.8 lakhs/mm3, serum creatinine- 2.92 mg/dl, Creatinine phosphokinase (CPK)- 568 IU/l, and lactate dehydrogenase (LDH)- 3824 IU/l. The peripheral smear showed normocytic normochromic anemia with no evidence of schistocyte. Urinalysis did not show any active sediments, but the dipstick test was positive for heme. His serum haptoglobin was <5 ng/ml, and iron stores, B12, and folate levels were within normal limits. After ruling out tropical infections and G6PD deficiency, as it was a Coombs-negative hemolytic anemia, the patient underwent flow cytometry for PNH evaluation, which revealed PNH clone in both RBC (CD55 deficiency- 84.5%, CD59 deficiency- 17.9%) and WBC (CD24, CD66B, FLAER, CD157, and CD14 deficiency- 73.6%, 75.6%, 76.6%, 77.6%, and 88.2%, respectively).
| 05/12/20 | 05/12/20 | 06/03/21 | 06/04/21 | 10/22/21 | 10/26/21 | 10/29/21 | 11/11/21 | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 3.3 | 7.3 | 8.1 | 7.4 | 7.5 | 7.9 | 10.1 | 11.8 |
| Total count/µl | 3400 | 5400 | 4300 | 4500 | 4300 | 4800 | 4400 | 4400 |
| Hematocrit (vol%) | 22.1 | 24 | 26 | 24 | 23.4 | 24.1 | 30.3 | 36.3 |
| Platelet count (lakhs/µl) | 1.7 | 1.50 | 1.7 | 1.8 | 2.5 | 1.93 | 2.00 | 2.10 |
| S. urea (mg/dl) | 225 | 45 | 32 | 40 | 37 | 20 | 19 | 14 |
| S. creatinine (mg/dl) | 18.4 | 0.9 | 1.9 | 2.0 | 2.92 | 2.06 | 1.46 | 0.97 |
| S. potassium (mmol/l) | 5.4 | 4.2 | 4 | 4.3 | 4.8 | 4.2 | 4.1 | 4.0 |
| LDH (U/l) | 280 | 3824 | 1006 | 400 |
AKI: acute kidney injury, LDH: lactate dehydrogenase, S.: Serum
Kidney biopsy [Figure 1] showed hemosiderin deposits within tubules. The patient was managed symptomatically and started on a low-dose steroid as per the hematologist’s advice. On follow-ups, his anemia had recovered completely, and he attained a nadir creatinine of 0.8 mg/dl.
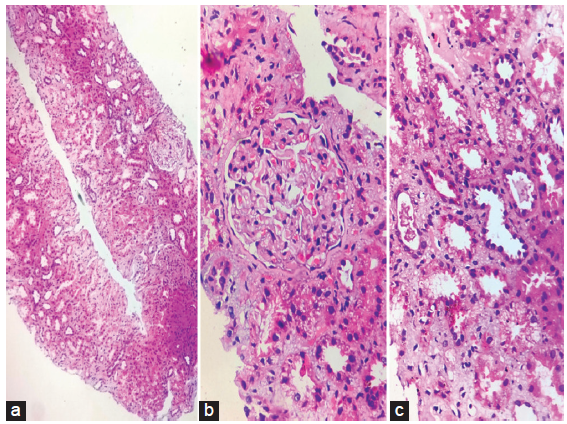
- (a) Hematoxylin and eosin staining of renal biopsy (10x) showing one normal glomerulus, dilated tubules and interstitial inflammation, (b) Golden brown pigment deposits (hemosiderin) in tubules (HE,300x), (c) Atrophic and dilated tubules with brown pigment and interstitial inflammation (HE,300x).
Discussion
The classical triad of hemolysis, venous thrombosis, and anemia with pancytopenia is rarely seen in PNH patients. Intravascular hemolysis can lead to hemoglobinuria, causing acute kidney injury (AKI) due to acute tubular necrosis (ATN).1 In a long-standing PNH, CKD can occur due to hemosiderosis and interstitial scarring. In a retrospective analysis, three renal manifestations of PNH were identified, that is, AKI, renal vein thrombosis, and Fanconi syndrome. CKD, however, was not seen.2 The most common cause of recurrent AKI in our country is tropical infections like malaria, scrub typhus, dengue, Leptospira, etc., followed by drug-induced allergic interstitial nephritis (AIN), renal stone disease, and urinary tract infections (UTIs). However, rhabdomyolysis and AKI due to intravascular hemolysis are rare causes. Recurrent episodes of AKI are associated with the development of CKD due to a reduction in nephron mass, vascular insufficiency, cell cycle disruption, and maladaptive repair mechanisms.3 Thakar et al.4 observed that individuals experiencing two or more episodes of AKI were more likely to progress to CKD. Even though supportive management is given initially, the only treatments for PNH are hematopoietic stem cell transplantation and eculizumab, a humanized monoclonal antibody against C5 that inhibits terminal complement activation.5 Hence, early recognition of this rare disorder and initiation of therapy can be game-changing in patients’ lives.
This report highlights the importance of considering rare causes for relapsing and remitting AKI cases. A holistic approach toward each patient brings out this possibility, thereby reducing the CKD burden in the country.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Transfer of glycosylphosphatidylinositol-anchored proteins to deficient cells after erythrocyte transfusion in paroxysmal nocturnal hemoglobinuria. Blood. 2004;104:3782-8.
- [CrossRef] [PubMed] [Google Scholar]
- Renal manifestations in paroxysmal nocturnal hemoglobinuria. Indian J Nephrol. 2017;27:289-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent acute kidney injury in tropics-epidemiology and outcomes. J Assoc Physicians India. 2017;57:72-04.
- [PubMed] [Google Scholar]
- Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury, anemia, and recurrent dark red urine. Am J Kidney Dis. 2019;74:A14-6.
- [CrossRef] [PubMed] [Google Scholar]







