Translate this page into:
An Unusual Glomerulopathy with Subepithelial Deposits Having Microspherular Structures Following COVID-19 Vaccine
Corresponding author: Tajamul Hussain Mir, Department of Nephrology, Government Medical College Associated Superspecialty Hospital, Srinagar, India. E-mail: thmir@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mir TH, Latief M, Sharma A, Jabeen B, Abbas F. An Unusual Glomerulopathy with Subepithelial Deposits Having Microspherular Structures Following COVID-19 Vaccine. Indian J Nephrol. doi: 10.25259/ijn_465_23
Abstract
We report an unusual glomerulopathy with nephrotic syndrome and acute kidney injury almost two weeks after the second injection of SARS CoV-2 vaccine covishield, ChAdOx1-nCoV-19 in a 75-year-old healthy man. Kidney biopsy revealed segmental stage 1 membranous glomerulopathy and collapsing focal segmental glomerulosclerosis pattern with immune complexes on IF. Electron microscopy (EM) revealed aggregates of spherular microparticles along glomerular capillary walls. After starting him on steroids, his kidney functions gradually improved with significantly reduced proteinuria. Our patient seems to be the only reported SARS CoV-2 vaccine-induced early membranous nephropathy showing a collapsing focal segmental glomerulosclerosis pattern and immune complexes on IF with subepithelial deposits consisting of microspherular particles on EM.
Keywords
SARS CoV-2 Vaccine Glomerulopathy
COVID-19
Subepithelial deposits with microspherular particles
FSGS
Membranous nephropathy
Introduction
Various autoimmune phenomena have been linked to SARS CoV-2 vaccines, including some common and uncommon glomerulopathies. We report the first case of SARS CoV-2 vaccine–induced collapsing glomerulopathy, a focal membranous nephropathy, with subepithelial deposits displaying microspherular structures, and AKI in an elderly male.
Case Report
A 75-year-old man presented for evaluation of a recent onset nephrotic syndrome with AKI. The only notable feature in his present history was recent exposure to a second dose of SARS CoV-2 vaccine Covishield, ChAdOx1-nCoV-19 at Serum Institute of India (adeno virus-vector vaccine) 2 weeks before the current illness, following which he had developed low-grade fever associated with aches, pains, and generalized edema. On examination, the patient had conjunctival pallor and anasarca. X-ray of the chest revealed bilateral pleural effusion. Investigations revealed a hemoglobin 10.5 g/dL, White blood cell count (WBC) 5,400/µL, Platelet count 170 x 103/µL, blood urea 170 mg/dL, serum creatinine 9.5 mg/dL, sodium 137 mEq/L, potassium 5.3 mEq/L, serum albumin 1.5 mg/dL, total protein 5 mg/dL, serum calcium (corrected) 8.5 mg/dL, uric acid 9.5 mg/dL, phosphorus 4.9 mg, cholesterol 270 mg/dL, and TG 160 mg/dL. Urine examination revealed protein 3+ and heme-granular casts, 24-hour total urine protein concentration was 10.5 g. Viral markers, vasculitis, and collagen vascular disease workup were negative. Kidney biopsy revealed segmental tuft sclerosis in several glomeruli with a few showing collapse of capillaries with remarkable hyperplasia of overlying visceral epithelial cells, exhibiting activated phenotype [Figure 1a]. Capillary loops showed patchy thickening. Severe acute tubular injury was seen with scattered hyaline and granular casts and sloughed epithelial cells in tubular lumina. DIF revealed IgG1 ++ IgG3 + IgG4 + C3 Kappa and Lambda ++ capillary wall granular (segmental) staining. IgA, IgG2, and C1q negative on IF study. IHC staining for known antigens associated with membranous nephropathy, anti-PLA2R (phospholipase A2 Receptor), thrombospondin (THSD7A), NELL-1, extosin-1, and semaphorin3B were negative in glomeruli. Transmission electron microscopy revealed a variable glomerular basement membrane (GBM) thickness from 321.6 to 669.5 nm. Significant foot process effacement was noted in 70–80% of visceral epithelial cells. Scattered (segmental) subepithelial deposits composed exclusively of spherular micro-particle aggregates, measuring 40–130 nm in diameter, were seen [Figure 1b arrowheads]. Given a strong temporal relationship with a recent COVID-19 vaccine (Covishield, ChAdOx1-S) in the background of multiple case reports in medical literature of various glomerular diseases following exposure to different COVID vaccines, a diagnosis based on morphological, immunopathological, and ultrastructural features, segmental stage 1 membranous glomerulopathy and collapsing FSGS, with immune complexes and aggregates of spherular microparticles along glomerular capillary walls distributed in a membranous pattern and an associated acute tubular injury complicating COVID-19 vaccine exposure was made. The patient was given three IV pulses of methyl prednisone 500 mg/day for 3 days followed by oral prednisone 1 mg/kg/day for 6 weeks followed by a gradual taper. Patient became dialysis independent on day 18 of hospitalization and at 14 weeks his tests revealed serum creatinine 1.8 mg, serum albumin 3.0 mg/dl, 24-hour urine protein 2 g. In the 16th week of treatment, he was readmitted with invasive pulmonary aspergillosis and ARDS requiring mechanical ventilation. He was managed with liposomal amphotericin-B and antibiotics. However, he died on day three of admission due to respiratory failure.
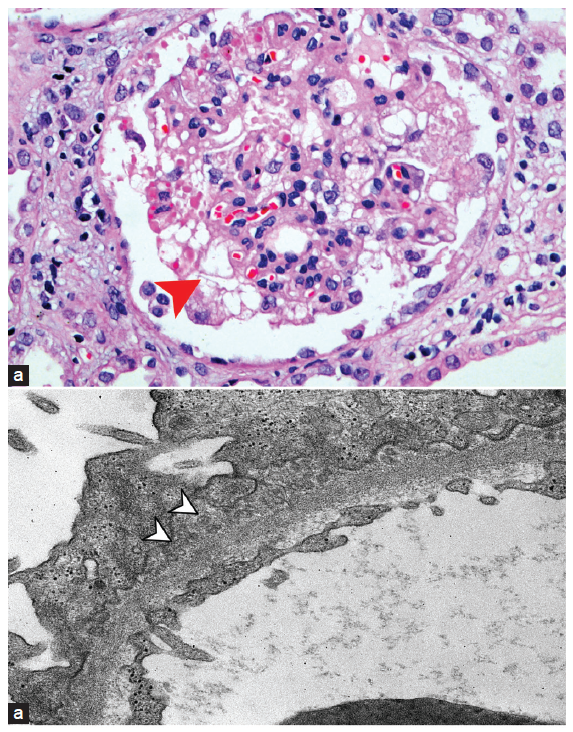
- (a) Photomicrograph showing collapse of a glomerular capillary tuft with hyperplasia of overlying visceral epithelial cells, which exhibit prominent cytoplasmic eosinophilic droplets (red arrowhead) (H & E × 200). (b) Transmission electron micrograph showing subepithelial deposits composed of aggregates of spherular microparticles (white arrow heads) (×8000).
Discussion
A massive global vaccination drive to curb COVID-19 has unveiled various autoimmune phenomena, including glomerulopathies ranging from IgA nephropathy, minimal change disease, collapsing glomerulopathy, membranous nephropathy to ANCA-positive paucimmune necrotizing glomerulonephritis that have been linked to different SARS CoV-2 vaccines in various case reports and series [Supplementary Material]. There has been a strong temporal relationship to both mRNA and adenovirus-vector-based SARS CoV-2 vaccines in these cases. This unique association reiterates the existence of a small but vulnerable population to this vaccine-induced/-triggered autoimmune phenomena.1,2 The mechanism behind this vaccine-induced de novo or relapsed glomerulonephritis has been postulated to be due to molecular mimicry of the spike protein antigen with host proteins in individuals with an underlying genetic susceptibility or particular HLA haplotypes.
Subepithelial deposits of unusual microspherular particles are a rare but distinctive finding in kidney biopsies. These microspherular particles have been identified in various renal diseases, especially membranous nephropathy. On electron microscopy, unlike the discrete, granular, and electron-dense deposits, present predominately on the subepithelial surface of the glomerular capillary basement membrane, these microspherular structures appear similar to organelles or as membrane-bound vesicles with electron-lucent cores measuring 35–150 nm in diameter. Microspherular particles are believed to be derived from nuclear pores following an autoantibody-induced breakdown or even from podocyte remnants. The presence of microspherular particles is an indicator of a secondary cause of MN.3,4,5 Vaccines, especially SARS-CoV-2 vaccines, are known to trigger a robust immune response, and in some genetically vulnerable patients, they could act as second hits to spark autoimmunity and its renal consequences.6 This is possibly the only reported case of COVID-19 vaccine–induced GN displaying early membranous nephropathy, a collapsing focal segmental glomerulosclerosis pattern, and immune complexes on IF with subepithelial deposits consisting of microspherular particles.
Conclusion
Various Glomerular lesions have been reported in relation to COVID19 infection and COVID19 vaccines, MN with Microspherular lesions have yet to be reported, making ours the first such observation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Glomerular disease in temporal association to SARS-CoV-2 vaccination - A series of 29 cases. Kidney 360. 2021;2:1770-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021;6:2969-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extracellular clusters of spherical microparticles in glomeruli in human renal glomerular diseases. Lab Invest. 1973;28:415-25.
- [PubMed] [Google Scholar]
- Membranous glomerulopathy with spherules: An uncommon variant with obscure pathogenesis. Am J Kidney Dis. 2006;47:983-92.
- [CrossRef] [PubMed] [Google Scholar]
- Subepithelial deposits with microspherular structures in membranous glomerulonephritis. Ultrastructural Pathology 2022:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccine-induced autoimmunity: The role of molecular mimicry and immune cross-reaction. Cell Mol Immunol. 2018;15:586-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







