Translate this page into:
Anti-C reactive protein antibodies in Indian patients with systemic lupus erythematosus
Address for correspondence: Dr. Vandana Pradhan, Department of Clinical and Experimental Immunology, National Institute of Immunohaematology, Indian Council of Medical Research, 13th Floor, King Edward Memorial Hospital, Parel, Mumbai - 400 012, Maharashtra, India. E-mail: pradhanv69@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Systemic lupus erythematosus (SLE) is characterized by over production of autoantibodies. C-reactive protein (CRP) is a phylogenetically highly conserved plasma protein that participates in the systemic response to inflammation. Anti-CRP antibodies might have biological functions of pathogenetic interest in SLE. We evaluated anti-CRP antibodies in Indian SLE patients and their association with anti-dsDNA antibodies and complement levels (C3 and C4). One hundred SLE patients diagnosed according to the American College of Rheumatology criteria were included. Disease activity was assessed using SLE disease activity index (SLEDAI). Anti-CRP autoantibodies were detected by enzyme linked immunosorbent assay. Anti-dsDNA antibodies were detected by indirect immunofluroscence test (Euroimmun Lubeck, Germany). High sensitivity CRP and complement levels (C3, C4) were detected using a Nephelometer. (BN ProSpec, Dade Behring, Germany). Anti-CRP antibodies were detected in 26% of SLE patients. Mean age of disease onset among anti-CRP positives was 22.4 ± 7.5, and 26.6 ± 9.3 years among anti-CRP negatives (P > 0.05). Anti-dsDNA positivity was significantly higher among anti-CRP positives (32.7%) as compared to anti-CRP negatives (16%) (P = 0.00519). No statistically significant difference was observed in SLEDAI scores of anti-CRP positive group and anti-CRP negative group (P > 0.05). We observed a positive correlation between anti-CRP antibodies and anti-dsDNA antibodies.
Keywords
Anti-C reactive protein antibodies
anti-dsDNA antibodies
high sensitivity C-reactive protein
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is characterized by abundant production of autoantibodies to a broad variety of structures including proteins of the cell cytoplasm and nucleus, cell membrane proteins, circulating proteins, nucleic acids (various DNAs and RNAs), and even phospholipids, lipid-protein structures, and glycosaminoglycans.[1] Hormonal and environmental factors as well as abnormal T cell and B cell function resulting in a wide range of autoantibodies and immune complexes (ICs) have been implicated in the etiopathogenesis of SLE.[2]
C-reactive protein (CRP) is a phylogenetically conserved protein that participates in the systemic response to inflammation.[3] CRP is synthesized as a non-glycosylated protein comprised of five identical non-covalently bound subunits with 206 amino acids (~23-kDa) arranged in cyclic symmetry around a central pore.[4] SLE patients can produce large amounts of CRP in response to bacterial stimulation and CRP levels can be used to differentiate a lupus flare from an infection.[1] CRP binds ICs and facilitates the clearance of soluble or particulate “debris” by means of phagocyte Fcγ receptors. Circulating autoantibodies against CRP (anti-CRP antibodies) are commonly found in SLE. It is not known whether these antibodies have any biological relevance, but considering the opsonic and complement-regulating properties of CRP, there are several pathogenetic implications.[2] It is not likely that the presence of anti-CRP antibodies explains the relative failure of CRP response in patients with active SLE. Instead, the possibility of post-translational modification of the CRP molecule by glycosylation could be relevant both with regard to clearance of circulating CRP and the induction of anti-CRP autoantibodies.[5]
This study was conducted to detect anti-CRP antibodies in Indian SLE patients and to evaluate their association with anti-dsDNA antibodies and complement levels (C3 and C4) to understand their immunopathogenic role.
Materials and Methods
This study was conducted in 100 SLE patients referred to our center at Mumbai, India over a 3 year period (2008-2010). Diagnosis was based on the 1997 American College of Rheumatology criteria.[6] The study was carried out after obtaining requisite ethics committee approval and a written consent from patients. Disease activity was assessed at the time of evaluation using SLE disease activity index (SLEDAI).[7] Pregnant and post-menopausal women, smokers, patients with diabetes and patients with significant hyperlipidemia were excluded. Lupus nephritis patients were classified according to World Health Organization criteria.[8] An enzyme linked immunosorbent assay (ELISA) protocol was developed as follows. Irradiated plates (Costar 3590, Cambridge, Massachusetts, USA) were coated with CRP from human plasma (Sigma catalogue No C-4063; St Louis, Missouri, USA) at a concentration of 10 mg/ml in TRIS-(Hydroxy Methyl Aminomethane (TRIS) buffer pH 7.4, 50 ml/well. The plates were incubated overnight at 4°C. They were then washed with 0.1% Tween/TRIS (wash solution) twice, blocked with 1% bovine serum albumin, 0.1% Tween/TRIS (block solution) 200 ml/well, and incubated for 1 h at room temperature. After washing 5 times with wash solution, 50 ml of serum diluted 1:50 in block solution were added to a well containing antigen plus block, and a well containing block only to control for reactivity to block. All were incubated overnight at 4°C. The plates were then washed 5 times with wash solution. Conjugate (Sigma A-3150 goat anti-human IgG alkaline phosphatase) was diluted 1:1000 in block solution, and 50 ml/well were added to each well. The plates were incubated for 1 h at room temperature and then washed 5 times with wash solution. Fifty microliters of substrate (p-nitrophenyl phosphate; sigma N2765 20 mg in 10 ml glycine buffer) were added to each well and incubated at room temperature for 1 h, 20 min. The optical densities (OD) were read on a plate reader at 405 nm. The difference between the OD in well with antigen plus block and the OD in well with block only was recorded as the result.
One hundred blood donors were tested by this method to determine the mean OD 2 SD for healthy individuals. Levels of anti-CRP above 2 SD, that is 0.9369 OD, were considered to be positive. IgG and IgM isotypes of anti-CRP autoantibodies were detected using alkaline phosphatase enzyme tagged IgG/IgM antibodies where para nitrophenyl phosphatase was used as a substrate. For confirmation of anti-CRP positivity and specificity of the anti-CRP method was determined by solid phase inhibition. A highly reactive serum was diluted fourfold from 1:25 to 1:6400 in TRIS buffer. Each dilution was added to a well-coated with antigen and one uncoated well. After overnight incubation at 4°C, the antibody fluids from plate 1 were transferred to a freshly coated plate (plate 2), which was incubated overnight at 4°C. The test was completed on plate 1 in order to obtain a baseline test result [Table 1]. Following overnight incubation the test on plate 2 was completed. Fluid phase inhibition was not successful.[2] Anti-dsDNA antibodies were detected by indirect immunofluroscence test using Crithidia luciliae as a substrate (Euroimmun Lubeck, Germany). High sensitivity CRP and serum complement levels such as C3, C4 were detected using a nephelometer. (BN ProSpec, Dade Behring, Germany). The laboratory was blinded to disease status of patients.
Statistical analysis
Continuous variables were expressed as mean + SD pairs of groups were compared using the student ‘t’ test for normally distributed continuous distribution. The ‘X2’ test was used for the categorical variables a needed. Statistical significance was set at P < 0.05.
Results
The prevalence of anti-CRP antibodies was 26%. IgG was the major isotype of immunoglobulin (46.1%) followed by IgM (23.1%). Both Immunoglobulin G (IgG) + Immunoglobulin M (IgM) were present in 20.3% of patients. Table 2 gives the details of female to male ratio, mean age. There was no statistically significant difference between the age of onset and age of evaluation between Anti-CRP positive patients and anti-CRP negative patients. Among anti-CRP positive patients, patient's ages at evaluation ranged between 10 years and 47 years (mean ± SD; 25.8 ± 8.0) as compared to anti-CRP negative patients that ranged between 13 years and 65 years (mean ± SD; 30.2 ± 9.9).

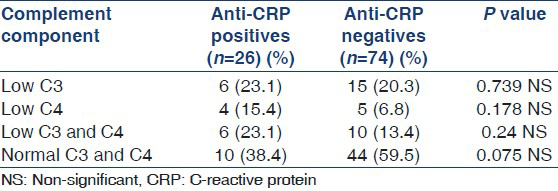
Anti-dsDNA (Titres > 1:160) antibodies were present in 32.7% patients among anti-CRP positives and 16% among anti-CRP negative patients (P = 0.00519). Anti-CRP antibody levels among anti-dsDNA positive and anti-dsDNA negative patients were 75.6 ± 27.9 u/ml and 70.2 ± 24.5 u/ml respectively. The cut-off value for anti-CRP negativity was set at <20 u/ml by testing sera of 100 normal healthy individuals. Among anti-CRP positives, 61.6% patients had reduced complement levels than anti-CRP negatives. There was no statistically significant difference noted when these two groups were compared (P = 0.06).
Based on the SLEDAI scores, SLE patients were grouped into mild (SLEDAI ≤ 8, n = 12), moderate (SLEDAI 9-18, n = 50) and severe (SLEDAI > 18, n = 38). No significant difference was observed in SLEDAI scores of anti-CRP positive group and anti-CRP negative group. There was no statistically significant difference for the presence of malar rash, photosensitivity, oral ulcer, arthritis, serositis, renal and hematological disorders when anti-CRP positives were compared with anti-CRP negatives (P > 0.05).
Discussion
SLE is a systemic autoimmune disease that affects multiple organ systems. Our study showed a prevalence of 26% for anti-CRP antibodies among Indian SLE patients studied which was similar to that reported by Rosenau (23%).[9] However some studies had reported a much higher incidence like 78% by Bell et al., in 39 out of 50 SLE patients and 40% by Sjowall et al.[110] IgG class autoantibodies are most common in SLE, but IgM class autoantibodies also occur.
In our study, a higher frequency of IgG antibodies to monomeric CRP was noted among anti-CRP positive SLE patients which was similar to the findings of Bell et al.[10] The occurrence of IgM subclass of anti-CRP antibodies among SLE patients in our study support the findings reported by Talal et al., indicating the possible mechanism for switching from IgM to IgG that bears an intimate relationship to disease severity.[111213] Although autoantibodies in SLE do not bind the native pentameric form of CRP, it cannot be excluded that they have pathogenetic implications, for instance by reacting with surface-bound CRP on cells and tissues. A statistically significant difference between anti-dsDNA and anti-CRP antibodies had also been reported by Sjöwall et al.[25]
Anti-CRP positive patients showed reduced complement levels in a higher number of patients as compared to anti-CRP negatives. Carvalho et al., reported that increased levels of anti-CRP antibodies correlate with lower levels of C3 and C4, suggesting complement consumption.[14] A statistically significant difference between anti-dsDNA antibodies and anti-CRP antibodies had already been reported by Sjowall et al.[25] Our study found higher titers of anti-ds-DNA antibodies in anti-CRP positive patients.
Source of Support: Indian Council of Medical Research
Conflict of Interest: None declared.
References
- Anti-CRP antibodies in systemic lupus erythematosus. Joint Bone Spine. 2010;77:384-9.
- [Google Scholar]
- Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2004;6:R87-94.
- [Google Scholar]
- CRP and anti CRP autoantibodies in systemic lupus erythematosus. Curr Rheumatol Rev. 2005;1:81-9.
- [Google Scholar]
- Pathogenic implications for autoantibodies against C-reactive protein and other acute phase proteins. Clin Chim Acta. 2007;378:13-23.
- [Google Scholar]
- Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- [Google Scholar]
- Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630-40.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-50.
- [Google Scholar]
- Autoantibodies to C-reactive protein (CRP) and other acute-phase proteins in systemic autoimmune diseases. Clin Exp Immunol. 1998;113:327-32.
- [Google Scholar]
- Immunologic regulation of spontaneous antibodies to DNA and RNA I. Significance of IgM and IgG antibodies in SLE patients and asymptomatic relatives. Clin Exp Immunol. 1976;25:377-82.
- [Google Scholar]
- C-Reactive protein and its implications in systemic lupus erythematosus. Acta Reumatol Port. 2007;32:317-22.
- [Google Scholar]
- Autoantibodies against monomeric C-reactive protein in sera from patients with lupus nephritis are associated with disease activity and renal tubulointerstitial lesions. Hum Immunol. 2008;69:840-4.
- [Google Scholar]
- Serum levels of autoantibodies against C-reactive protein correlate with renal disease activity and response to therapy in lupus nephritis. Arthritis Res Ther. 2009;11:R188.
- [Google Scholar]







