Translate this page into:
Anti-Glomerular Basement Membrane Antibody Disease: Clinicopathologic Profile and Outcomes
Corresponding author: Manoj Kumar, Institute of Nephrology, Madras Medical College, Chennai, Tamil Nadu, India. E-mail: manoj.k.king@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar M, Jayaprakash V, Gopalakrishnan N, Dineshkumar T, Sakthirajan R, Dhanapriya J. Anti-Glomerular Basement Membrane Antibody Disease: Clinicopathologic Profile and Outcomes. Indian J Nephrol. 2025;35:265-9. doi: 10.25259/IJN_110_2024
Abstract
Background:
Anti-glomerular basement membrane antibody disease is a rare autoimmune disease caused by antibodies to α3 chain of type 4 collagen. Patients presenting with severe renal involvement requiring dialysis have poor response to treatment.
Materials and Methods:
We conducted a retrospective and prospective study at Institute of Nephrology, Madras Medical College, Chennai, India by analyzing the data of patients with biopsy-proven anti-GBM antibody disease treated from January 2013 to December 2019.
Results:
There were 2,949 kidney biopsies in the study period and 92 showed crescentic glomerulonephritis (GN). Of those, 20 patients (10 males) had anti-GBM antibody disease. Mean age was 40.75 ± 14.75 years. Rapidly progressive renal failure was the most common mode of presentation (95%); five (25%) patients had diffuse alveolar hemorrhage (DAH) and nineteen patients (95%) required dialysis at presentation. Seven patients (35%) were positive for anti-neutrophil cytoplasmic antibody (anti-myeloperoxidase in six and anti-proteinase 3 in one). Of the twelve patients (60%) who received immunosuppression (cyclophosphamide, steroids, and plasma exchange), two patients (10%) attained remission, and two patients (10%) expired due to sepsis. Crescentic GN was the predominant pathology in kidney biopsy in 19 patients (95%). Mesangial hypercellularity with deposition of IgA and C3 in mesangium was present in one patient.
Conclusion:
In our study, anti-GBM antibody disease accounted for 21.7% of crescentic GN. Majority of patients presented late, requiring dialysis. Patient survival was 90%, while renal survival was only 10%. One patient had co-occurrence of IgA nephropathy with anti-GBM antibody disease.
Keywords
Anti-glomerular basement membrane disease
Crescentic glomerulonephritis
Goodpasture syndrome
Introduction
Anti-glomerular basement membrane (GBM) antibody disease is a rare autoimmune disease caused by antibodies to α3 chain of type IV collagen. It presents either as isolated renal involvement, which can range from mild to severe, or as goodpasture syndrome (GPS) with renal involvement and diffuse alveolar hemorrhage (DAH).1 When diagnosed early and treated with cyclophosphamide (CYC), steroids, and plasma exchange (PLEX), renal survival is 95%.2 Data about clinical presentation and outcomes of patients with anti-GBM antibody disease in India are limited. We present a case series from a tertiary care center in southern India.
Materials and Methods
We conducted a retrospective and prospective observational study, from clinical details of patients with biopsy-proven GBM antibody disease at the Institute of Nephrology, Madras Medical College from January 2013 to December 2019. IEC approval and appropriate patient consent was obtained. We collected and analyzed data on demographic profiles, clinical presentations, laboratory values, anti-neutrophil cytoplasmic antibody (ANCA) serology, anti-GBM antibody serology, hemodialysis, plasmapheresis, immunosuppressive therapy, and clinical outcomes. All kidney biopsy specimens underwent light microscopy (LM) and immunofluorescence (IF) study.
The immunosuppression protocol consisted of pulse intravenous methylprednisolone followed by oral prednisone (PDN) at a dosage of 1 mg/kg body weight for 1 month, followed by tapering. For anti-GBM antibody disease, patients received intravenous CYC at a dosage of 500 mg monthly for 3 months. For ANCA-positive patients, the dosage was 15 mg/kg at 0, 2, 4, 7, 10, and 14 weeks. In addition, patients underwent alternate-day PLEX at a volume of 35–40 mL/kg body weight with plasma or human albumin replacement. Immunosuppression was discontinued in patients who remained dialysis-dependent at 3 months.
At 1-year follow-up, outcomes were classified as follows: Complete remission: dialysis-independent with serum creatinine <1.5 mg/dL and no lung hemorrhage; Partial remission: dialysis-independent but serum creatinine >1.5 mg/dL and no lung hemorrhage; Treatment failure: dialysis-dependent; and death.
Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as mean with standard deviation or median with interquartile range, based on the normality of the distribution. The Chi-square test was used for categorical variables, and the t-test was used for continuous variables, with a p-value < 0.05 considered significant.
Results
There were 2,949 kidney biopsies done from 2013 to 2019. Crescentic glomerulonephritis (GN) was present in 92 patients. Of those, 20 patients had anti-GBM antibody disease. Ten patients (50%) were males. The mean age was 40.75 ± 14.75 years (range 19–61 years). Hypertension was present in 19 patients (95%). The median duration of symptoms was 15 days (IQR 7–20.5). Rapidly progressive renal failure (RPRF) was the most common mode of presentation (n = 18, 90%), followed by edema and frothy urine in one patient and macroscopic hematuria and anorexia in another (5%). Two patients had diabetes, and one patient had hepatitis C infection. Five patients (25%) were smokers, and one (5%) had occupational exposure to silica. Mean serum creatinine was 11.41 ± 5.14 mg/dL (range 1.9–23.4) and hemoglobin was 7.47 ± 2.28 g/dL. Anti-GBM antibody was positive in all patients. Baseline characteristics, kidney pathology, and outcomes of patients are given in Table 1.
| Age | Sex | Presentation | DAH | Creatinine (mg/dl) | ANCA | % crescents/ %sclerosis in glomeruli | IS | Outcome |
|---|---|---|---|---|---|---|---|---|
| 30 | M | RPRF | Yes | 19.6 | Negative | 100/- | Yes | Expired (sepsis) |
| 55 | M | RPRF | Yes | 16.8 | MPO | 100/- | Yes | DD; DAH recovered |
| 55 | F | RPRF | No | 9.6 | Negative | 37/63 | No | DD |
| 28 | F | RPRF | Yes | 7.8 | PR3 | 32.5/63.5 | Yes | DD; DAH recovered |
| 55 | M | RPRF | No | 8.9 | MPO | 100/- | Yes | DD |
| 19 | M | RPRF | No | 8.9 | MPO | 100/- | Yes | DD |
| 23 | F | RPRF | No | 9.8 | MPO | 100/- | Yes | Expired (sepsis) |
| 44 | F | RPRF | No | 7.9 | MPO |
12.5/- Granuloma seen around necrotic glomeruli |
Yes | Partial recovery. Creatinine 3.4 mg/dl. |
| 60 | M | RPRF | No | 7.2 | Negative |
88.5/- Diffuse and nodular mesangial expansion in all glomeruli |
No | DD |
| 55 | F | RPRF | No | 6.3 | MPO | 61/35 | Yes | DD |
| 50 | M | RPRF | No | 15 | Negative | 100/- | No | DD |
| 57 | M | Edema, frothy urine | Yes | 1.9 | Negative | 100/- | Yes | Partial recovery. Serum creatinine 1.7 mg/dl |
| 26 | F | RPRF | Yes | 18.4 | Negative | 100/- | Yes | DD; DAH recovered |
| 19 | F | Hematuria, anorexia | No | 9.3 | Negative | -/100 | No | DD |
| 22 | F | RPRF | No | 14.5 | Negative |
81/19 Fibrous crescents |
No | DD |
| 36 | M | RPRF | No | 23.4 | Negative |
43/57 IFTA 60% |
No | DD |
| 61 | M | RPRF | No | 9.3 | Negative | 88/12 | No | DD |
| 40 | F | RPRF | No | 10.7 | Negative |
81/- 50% cellular, 25% fibrocellular, and 25% fibrous crescents |
No | DD |
| 35 | M | RPRF | No | 12.6 | Negative |
60/40 Mesangial hypercellularity and matrix expansion. IgA & C3 in mesangium in IF |
Yes | DD |
| 45 | F | RPRF | No | 10.3 | Negative | 78/- | Yes | DD |
ANCA: Antineutrophil cytoplasmic antibody, DAH: Diffuse alveolar hemorrhage, DD: Dialysis-dependent, IS: Immunosuppression, MPO: Myeloperoxidase, PR3: Proteinase - 3, RPRF: Rapidly progressive renal failure, IF: Immunofluorescence.
Five patients (25%), three males and two females, had DAH (hemoptysis and/or CT evidence of alveolar hemorrhage). Three of them had exposure to lung irritants (smoking = 2, silica exposure = 1) although the association was not statistically significant (p = 0.09).
In kidney biopsy, crescentic GN was the predominant pathology in 19 (95%) patients – cellular crescents in 14 (70%) and fibrocellular to fibrous crescents in 5 patients (25%). One patient (5%) had global glomerulosclerosis. Segmental fibrinoid necrosis of glomerular tuft was present in four (20%) biopsies. Vasculitis with granuloma formation was present in one patient who tested positive for anti-myeloperoxidase (MPO) ANCA. One patient, a 60-year-old male with diabetes, had diffuse nodular mesangial expansion in LM suggestive of diabetic nephropathy. IF showed linear IgG deposition over glomerular capillaries in all biopsies. One patient, a 35-year-old male with untreated hypertension for 2 years and presented with RPRF, had IgA and C3 deposition in the mesangium suggestive of coexistent IgA nephropathy [Figure 1].
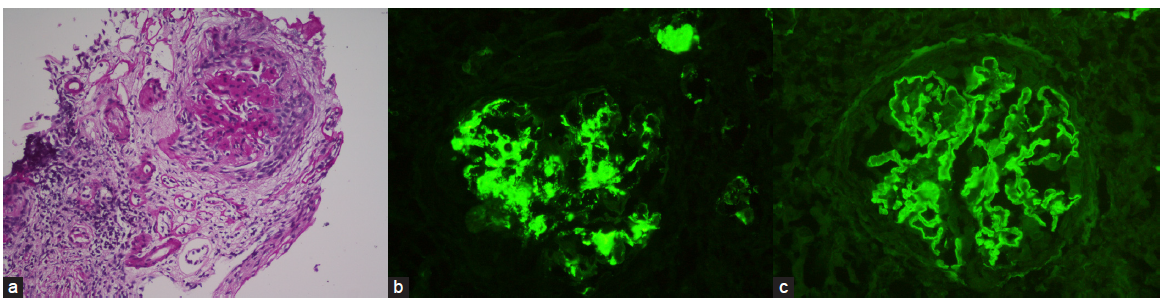
- (a) Kidney biopsy of patient with anti-GBM antibody disease with IgA nephropathy. Light microscopy (40x magnification) with periodic acid schiff stain shows a glomerulus with mesangial hypercellularity and matrix expansion and crescent formation. (b) IgA deposition in mesangium. (c) IgG linear deposition along glomerular basement membrane.
Seven patients (35%) tested positive for ANCA, with anti-MPO detected in six (30%) and anti-proteinase 3 (PR3) in one patient (5%). All ANCA-positive patients required dialysis at presentation. Two patients had DAH. Immunosuppression was given to all seven patients but only one patient (14.2%) attained partial remission and became dialysis-independent with a creatinine of 3.4 mg/dL. One patient expired due to sepsis.
Twelve patients (60%) received immunosuppression including seven patients with ANCA disease, three with DAH, and one patient with co-existent IgA nephropathy. Maintenance therapy with prednisone and azathioprine was given to one patient with ANCA disease, who attained partial remission. At follow-up, sixteen patients (80%) remained dialysis-dependent, two patients (10%) attained partial remission, and two patients (10%) expired due to sepsis. No patient attained complete remission.
Discussion
We did a retrospective and prospective observational study of patients with anti-GBM antibody disease over a 7-year period. Previous studies have described a bimodal age distribution with pulmonary-renal involvement in the third decade and isolated renal involvement in the sixth to seventh decades.3 Previous Indian studies have reported a mean age of 33–44.8 years [Table 2].4–7 In our study, mean age was 40.75 ± 14.75 years. Sex distribution of patients with anti-GBM antibody disease has varied from male predominance to equal to slight female predominance.8–10 In India, two older studies showed >80% male predominance, while the recent studies had slightly equal sex distribution.4–7 We noted a 1:1 sex distribution in our patients.
| Muthukumar et al. (1988–1999)4 | Ahmed et al. (2002–2004)5 | Prabhakar et al. (2013–2014)6 | Parikh et al. (2011–2017)7 | Current study (2013–2019) | |
|---|---|---|---|---|---|
| Number of patients | 12 | 18 | 17 | 25 | 20 |
| Age (years)* | 38.7 ± 13.9 | 33 ± 13.2 | 39.11 ± 16.58 | 44.8 ± 17.66 | 40.75 ± 14.75 |
| Males n (%) | 10 (83.3) | 16 (88.9) | 9 (52.9) | 11 (44) | 10 (50) |
| Symptom duration (days)# | 19.5 | 42.8 ± 38.6* | N/A | N/A | 15 (7 – 20.5) |
| DAH n (%) | 4 (33.3) | 6 (33.3) | 4 (23.5) | 7 (28) | 5 (25) |
| Lung irritant exposure n (%) | |||||
| Smoking | 4 (33.3) | 7 (38.9) | 5 (29) | N/A | 5 (25) |
| Others | 1 (8.3) petroleum sniffing |
1 (5) Silica (quarry worker) |
|||
| Mean creatinine mg/dL | 6.9 ± 3.2 | 9.89 ± 3.49 | 8.64 ± 5.29 | 12.088 ± 5.17 | 11.41 ± 5.14 |
| Dialysis requirement | N/A | 17 (94.3) | 14 (82.3) | 24 (96) | 19 (95) |
| ANCA-positive n (%) | 1 (8.3) | 1 (5) | 4 (23.5) | 12 (48) | 7 (35) |
| ST/CYC/PLEX n (%) | 5 (41.7) | 7 (44) | 16 (94.1) | 14 (76) | 12 (60) |
| Remission n (%) | |||||
| Total (out of those who received therapy) | 3 (60) | 4 (57.1) | 4 (25) | 1 (7.1) | 2 (16.7) |
| Dialysis-independent patients | N/A | 2/2 (100) | 3/3 (100) | 1/1 (100) | 1/1 (100) |
| Dialysis-dependent patients | N/A | 2/16 (12.5) | 1/16 (6.3) | Nil | 1/19 (5.2) |
*Mean with standard deviation. #Median with IQR. ANCA: Antineutrophil cytoplasmic antibody, CYC: Cyclophosphamide, DAH: Diffuse alveolar hemorrhage, N/A: Not available, PLEX: Plasma exchange, ST: Steroids, IQR: Interquartile range.
Between 40% and 60% of patients with anti-GBM antibody disease have DAH which can even precede renal involvement by weeks to months. Exposure to lung irritants like cigarette smoke, inhaled hydrocarbons, or respiratory infections act as potential triggers by damaging the alveolar basement membrane and exposing the antigen.11 Prevalence of DAH in Indian studies is lower, only 23.5% to 33.3%, probably due to lower number of smokers in the study population.6 In our study, DAH occurred in 25% of patients. We didn’t find any statistically significant association with exposure to lung irritants.
In a large biopsy series, anti-GBM antibody disease accounts for 15–20% of cases with crescentic GN.12 The hallmark pathology of anti-GBM antibody disease, crescentic GN (crescents in >50% glomeruli), is seen >90% of patients and half of them have crescents in >85% of glomeruli.3 In our study, anti-GBM antibody disease accounted for 21.7% of crescentic GN. Ninety-five percent of our patients had crescents in >50% glomeruli.
Levy et al. reported ANCA positivity in 32% of anti-GBM antibody disease patients, predominantly anti MPO (82%), with 1 year patient and renal survival to be 52% and 26%, respectively.13 Likewise, Prabhakar et al. had ANCA in 23% of patients and the patient and renal survival at 1 year were 75% and 50%, respectively.6 Thirty-five percent patients in our study were ANCA-positive, with patient and renal survival of 85.8% (one patient died due to sepsis) and 14.2%, respectively.
The co-occurrence of IgA nephropathy along with anti-GBM antibody disease is rare and the causal relationship between the two is still unclear.14 It is hypothesized that the inflammatory injury to glomerulus from IgA nephropathy can trigger anti-GBM antibody disease.15 In our patient who had hypertension for 2 years prior to presentation and kidney biopsy showed evidence of IgA nephropathy in LM along with crescentic GN, the temporal relationship cannot be clearly established as he did not undergo any laboratory evaluation or kidney biopsy at the initial diagnosis of hypertension.
Proportion of patients requiring dialysis at presentation is higher in Indian studies (82.3–96%) and is attributed to delay in seeking care (median duration of symptoms 19.5–42.38 days). The severity of disease at presentation directly affects renal survival, with 1 year renal survival of 95% when serum creatinine <5.7 mg/dL, 82% in those when >5.7 mg/dL but not requiring dialysis, and only 8% for those requiring dialysis in the Hammersmith study.2 Whereas in previous Indian studies the remission rates were 100% for patients not requiring dialysis vs. 6.3–12.5% in those who did. Even with newer therapeutic options like Rituximab or IgG degrading enzyme of Streptococcus pyogenes (IDeS), patients with late presentation requiring dialysis and/or chronic changes in biopsy did not attain remission.16,17 This underscores the necessity to recognize anti-GBM antibody disease early for timely initiation of therapy to improve renal survival. Ninety-five percent of patients required dialysis at presentation in our study. Remission rate was 5.2% (1/19) among patients requiring dialysis and 100% (1/1) amongst those who did not.
Our study has certain limitations. It is a single-center study. Since this is a case series, we did not analyze prognostic factors but it was evident that patients requiring dialysis at presentation had poor renal outcomes.
In our retrospective and prospective study, anti-GBM antibody disease accounted for 21.7% of crescentic GN. Majority of patients presented late, requiring dialysis. Patient survival was 90%, while renal survival was only 10%. One patient had co-occurrence of IgA nephropathy with anti-GBM antibody disease. Early diagnosis of anti-GBM antibody disease is critical to improve renal survival.
Conflicts of interest
There are no conflicts of interest.
References
- Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033-42.
- [CrossRef] [PubMed] [Google Scholar]
- Anti–Glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am J Clin Pathol. 2006;125:445-50.
- [CrossRef] [PubMed] [Google Scholar]
- Anti glomerular basement membrane disease antibody disease – A disease with late presentation, diagnosis and potentially fatal outcome. Indian J Nephrol. 2000;10:99-100.
- [Google Scholar]
- Anti glomerular basement disease - An Indian scenario. 2004;14:182-6.
- Anti-glomerular basement membrane disease: Case series from a tertiary center in North India. Indian J Nephrol. 2017;27:108.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-glomerular basement membrane disease: a case series from a single center in Western India. J Egypt Soc Nephrol Transplant. 2021;21:31.
- [CrossRef] [Google Scholar]
- goodpasture’s syndrome: An analysis of 29 cases. Kidney Int. 1978;13:492-504.
- [CrossRef] [PubMed] [Google Scholar]
- Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. BMJ. 1986;292:301-4.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics and outcome of anti-glomerular basement membrane disease: A single-center experience. Ren Fail. 1996;18:105-12.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-GLOMERULAR basement membrane disease. Clin J Am Soc Nephrol. 2017;12:1162-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-glomerular basement membrane disease. Kidney Int. 2003;64:1535-50.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66:1535-40.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-glomerular basement membrane disease with IgA nephropathy: A case report. World J Clin Cases. 2022;10:3916-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of anti-glomerular basement membrane glomerulonephritis during the course of IgA nephropathy: a case report. BMC Nephrol. 2019;20:25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rituximab for the treatment of refractory anti-glomerular basement membrane disease. Ren Fail. ;44:1123-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IdeS in anti–glomerular basement membrane disease: Is this the new deal? Kidney Int. 2019;96:1068-70.
- [CrossRef] [PubMed] [Google Scholar]








