Translate this page into:
Anti-glomerular basement membrane disease: Case series from a tertiary center in North India
Address for correspondence: Dr. M. Rathi, Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012, India. E-mail: drmanishrathi2000@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anti-glomerular basement (anti-GBM) disease is an uncommon disorder with a bimodal age of presentation. Patients presenting with dialysis-dependent renal failure have poor renal outcomes. There is limited data regarding the clinical presentation and outcomes of anti-GBM disease from India. We conducted this prospective study to analyze the clinical presentation and outcomes of anti-GBM disease at a large tertiary care hospital in North India over 1½ years. Subjects with a biopsy proven anti-GBM disease (light microscopic examination showing crescents and immunofluorescence examination showing linear deposition of IgG) with or without positive anti-GBM antibodies in serum were included in the study and followed-up for at least 12 months. All the patients were treated with steroids, cyclophosphamide, and plasma exchange. A total of 17 patients (nine males) were included. The mean age at presentation was 39.11 ± 16.58 (range 11–72) years. Twelve patients (70%) presented with rapidly progressive glomerulonephritis (RPGN), 4 (23.5%) presented with Goodpasture syndrome, while 1 (5.8%) had nephritic syndrome, 7 (41%) were hypertensive, and 14 (82.3%) required dialysis at the time of presentation. Four patients (23.5%) had associated anti-neutrophil cytoplasmic antibody positivity (anti-myeloperoxidase antibodies in all). Fourteen (87.5%) patients had crescentic glomerulonephritis, while 5 (31.25%) showed necrotizing (n = 4) or granulomatous (n = 1) in the vasculitis. Of 16 patients who received treatment, four (23.25%) achieved complete remission. In this single-center study, the majority of anti-GBM disease patients presented with RPGN and had crescentic glomerulonephritis on biopsy with poor treatment outcome.
Keywords
Anti-glomerular basement disease
crescentic glomerulonephritis
Goodpasture syndrome
rapidly progressive glomerulonephritis
Introduction
Anti-glomerular basement (anti-GBM) disease is an uncommon condition with a bimodal age of presentation.[1] It presents either as a renal limited disease, with manifestations varying from relatively mild renal insufficiency to rapidly progressive glomerulonephritis (RPGN); or in the form of Goodpasture syndrome (GPS) which includes RPGN and diffuse alveolar hemorrhage (DAH). Almost 50% of patients present with dialysis-requiring renal failure,[2] and these patients have 1-year patient and renal survival of about 65% and 8%, respectively.[3] Intensive treatment in the form of plasma exchange, cyclophosphamide (CYC), and steroids has been shown to improve the outcomes.[3] It has been described that the double positive disease (anti-GBM and anti-neutrophil cytoplasmic antibodies; ANCA) has a poor prognosis and behave like anti-GBM disease rather than ANCA-associated vasculitis.[4] There is limited data on clinical presentation and treatment outcome of anti-GBM disease from India.
Materials and Methods
This was a prospective study conducted between January 2013 and June 2014 in the Department of Nephrology at a large tertiary care hospital in North India. All patients presenting with RPGN or GPS with a biopsy proven anti-GBM disease (immunofluorescence [IIF] examination showing linear deposition of IgG) with or without anti-GBM antibodies in serum were included in the study and followed prospectively for at least 12 months. Patients were followed monthly for initially 3 months, followed by quarterly review until the end of follow-up. DAH was diagnosed by either high-resolution computed tomography scan of the chest or clinical criteria, such as a drop in hemoglobin of ≥1g/dl over 24 h with a cough, respiratory distress, or hemoptysis.
Anti-GBM antibody assay was performed at the time of presentation by enzyme-linked immunosorbent assay (ELISA), and results were provided as optical density. The cut-off of 0.29 was taken as reference. ANCA assay was performed in all patients by both IIF and ELISA methods to ascertain double positive cases.
Histopathological examination
All patients except one who had associated chronic liver disease and coagulopathy underwent kidney biopsy. The diagnosis in a patient not subjected to renal biopsy was made on the basis of clinical presentation of GPS and positive anti-GBM antibodies. All biopsy specimens were examined by a single pathologist (RN). Light microscopic and IIF examination were performed on all biopsy specimens and looked for the presence of crescents, fibrinoid necrosis, glomerular sclerosis, vasculitis, interstitial inflammation, and interstitial fibrosis and tubular atrophy.
Treatment and outcome
All patients were treated with steroids in the form of intravenous methylprednisolone 500 mg once a day for 3 days followed by 1 mg/kg prednisolone for 6 weeks, followed by the taper. In addition, all patients were also prescribed oral CYC at a dose of 2 mg/kg/day, except in those with associated ANCA positivity or evidence of vasculitis on renal biopsy, where the intravenous monthly pulse (15 mg/kg) of CYC was prescribed. Patients presenting with DAH or dialysis-requiring renal failure were also given daily exchange of plasmapheresis with the exchange of 40 ml/kg of plasma for 10–14 sessions or until improvement, whichever was earlier.
Outcome was defined as (1) complete clinical remission: Serum creatinine (SCr) <1.5 mg/dl and no pulmonary hemorrhage; (2) partial remission: SCr ≥1.5 mg/dl but dialysis independent, no pulmonary hemorrhage; (3) treatment failure: Dialysis dependent or continued pulmonary hemorrhage,[5] (4) death.
Results
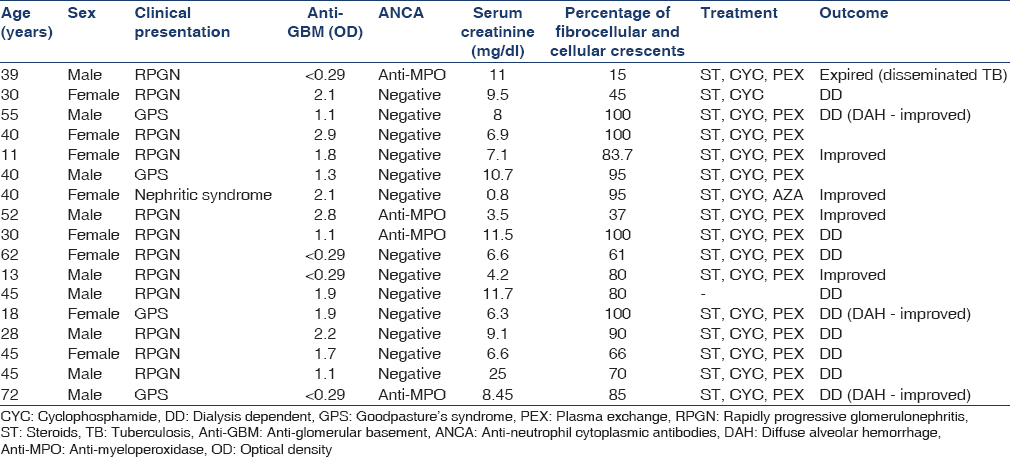
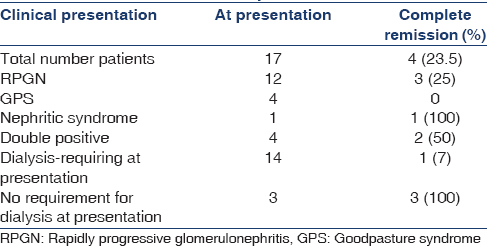
Seventeen patients (nine males and eight females) were included in the study. The baseline characteristics are summarized in Table 1, while the case details are summarized in Table 2. The majority were young at presentation with a mean age of 39.11 ± 16.58 (11–72) years. Twelve patients (70%) presented with RPGN, four patients (23.5%) presented with GPS, and one patient (5.8%) presented with acute nephritic syndrome. Fourteen patients (82.3%) required dialysis at presentation. Thirteen patients (76.4%) had positive anti-GBM antibody, of which 4 (23.5%) patients also had associated ANCA positivity. All these four patients had perinuclear-ANCA on IIF and anti-myeloperoxidase [anti-MPO] antibodies by ELISA.

Histopathology
Fourteen (87.5%) of 16 patients had crescentic glomerulonephritis (i.e. ≥50% of glomeruli showed crescents). Five patients (31.25%) had evidence of vasculitis on biopsy in the form of necrotizing inflammation (n = 4) or granulomatous inflammation (n = 1) in the media of small arteries. Of these five patients, only one patient had associated positive ANCA. Glomerular tuft necrosis was seen in five patients (31.25%), of which four had evidence of vasculitis and one patient was ANCA positive.
Outcome
A total of 16 patients received treatment. Seven (43.7%) of 16 patients received intravenous CYC, while the rest received oral CYC. In addition, 14 patients (87.5%) also received plasma exchange. One patient with decompensated chronic liver disease died with sepsis before the initiation of treatment. Four patients (23.25%) achieved complete remission, of which three achieved it within 3 months, while one patient took 5 months to achieve the same. Out of 14 patients requiring dialysis at presentation, complete remission was seen in only 1 (7%), while all three nondialysis requiring patients achieved complete remission. DAH improved in all the four patients presenting with GPS, however, none of them showed renal recovery. Of the 12 patients with RPGN, 3 (25%) showed complete remission, while one patient with nephritic syndrome also achieved complete remission. The treatment outcomes are shown in Table 3.
Double positive disease: Four patients (23.5%) had the double positive disease. Two patients presented with severe disease (dialysis-dependent renal failure), one with nephritic syndrome, and other patient presented with nondialysis requiring renal dysfunction. Patients with less severe disease (n = 2) achieved complete remission.
Two (11.7%) of 17 patients died; one with disseminated tuberculosis and other with decompensated liver disease. One patient undergone kidney transplantation with normal graft function until the end of follow-up.
Discussion
In this study, we recruited and followed 17 patients with anti-GBM disease, of which four had double positive disease (anti-GBM and ANCA). Fourteen patients required dialysis (82.3%) at presentation and four presented with GPS.
Retrospective studies have shown that the peak incidence of anti-GBM antibody disease seems to be in the third decade with predominance in males, and a second peak in the sixth and seventh decades affecting men and women equally.[1] A previous study from India has shown that the mean age of onset was 33.4 ± 13.2 years with male predominance (16:2).[6] In our study, the mean age of presentation was 39.11 ± 16.58 (11–72) years, and there was an equal gender distribution (male: female = 9:8) which is similar to the study by Fischer and Lager[7] which also showed an equal gender distribution (M: F = 1:1.35). In our cohort, 82.3% of the patients required dialysis at presentation, compared to 50% incidence reported in literature.[2] Poor awareness among patients and primary health care providers leading to late referral may explain this finding. Lazor et al. have reported that 60–70% patient had pulmonary involvement, particularly alveolar hemorrhage,[8] while Ahmad et al. noted hemoptysis in 33% patients.[6] Twenty-five percent of our patients had DAH, which is lower than the previous studies. Only five patients (29%) were smokers in our cohort, which may explain the low incidence of pulmonary hemorrhage. Thirteen patients (76%) were positive for anti-GBM assay. ELISA assays using native or recombinant human alpha-3 (IV) antigen substrates tend to have sensitivity and specificity up to 95–100%.[9] We have used recombinant human antigens for the assay, however, the sensitivity was low. Low antibody titers may explain this finding. In contrast to study by Fischer and Lager,[7] which showed necrotizing glomerulitis in 88% of the patients, we found tuft necrosis in only 35%. Except for one patient, other patients either had evidence of small vessel vasculitis on biopsy or had ANCA positivity. However, the number of glomeruli with crescents was almost similar in both studies (88% vs. 87.5%). Five of 16 patients had evidence of vasculitis on biopsy. Glomerular fibrinoid necrosis and inflammation occasionally extend into the most proximal part of contiguous hilar arterioles; otherwise, acute inflammation of renal vessels, other than glomerular capillaries, is not typical for anti-GBM glomerulonephritis unless the patient has concomitant ANCA.[10] In our cohort, out of five patients with evidence of vasculitis, only two had positive ANCA. The reason might be either low titers of these antibodies or the epitope diversity of ANCA.[11] However, de novo vasculitis is very unlikely in anti-GBM disease as the antigen is not expressed in blood vessels. In our study, 16 of 17 patients (94%) received immunosuppression in the form of steroids and CYC and 14 patients (82%) received plasmapheresis. In a study by Ahmad et al.,[6] only seven patients (39%) received adequate treatment in the form of plasma exchange and immunosuppression, and the reason behind the same is not forthcoming. Alchi et al. treated 74% of their patient with immunosuppression and plasma exchange, and noted overall 1-year patient and renal survival to be 88 and 16%, respectively.[12] Levy et al.[3] reported a retrospective cohort of 71 patients treated with plasma exchange and identical immunosuppressive regimes. Patients presenting with an SCr <500 μmol/L (5.7 mg/dl) had 100% patient survival and 95% renal survival at 1-year. In those with dialysis-dependent presentation, these values were 65% and 8%, respectively. These findings are similar to our study. Fourteen patients (82%) needed dialysis at presentation and at 1-year patient and renal survival were 85% and 7%, respectively, while those patients who did not require dialysis (n = 3) at presentation have 100% patient and renal survival at 1-year. The overall patient and renal survival in our study was 88% and 23%, respectively, at the end of follow-up. About 20–30% of the anti-GBM patients have been shown to have associated ANCA positivity and majority are anti-MPO positive.[412] Levy et al. showed that 5% of all ANCA-positive serum samples were also positive for anti-GBM antibodies, and 32% of all anti-GBM positive samples had detectable ANCA, and 82% had anti-MPO-ANCA. Patient and renal survival rates were 52% and 26%, respectively, at 1-year. Sixty-eight percentage of patients were dialysis dependent at presentation, and none of these recovered renal function, despite immunosuppression with or without plasma exchange.[4] Rutgers et al.[13] reported no significant difference in the 1-year patient survival in those with anti-GBM (100%), double positive (79%), and MPO-ANCA vasculitis (75%). In our study, 23% of the patients had ANCA positivity, and patient and renal survival were 75% (one patient died of disseminated tuberculosis) and 50%, respectively. Patients who were dialysis dependent did not recover which is consistent with the study by Levy et al.[4] A comparison of our study with one case series published previously from India has been summarized in Table 4.

The results of our study should be interpreted in light of several limitations. We measured anti-GBM antibodies semiquantitatively in the serum which hindered estimation of correlation of anti-GBM antibody titers with prognosis. Moreover, the assay was not repeated after the treatment. This was a case series, and we did not look for any prognostic markers, however, it is evident from this study that dialysis-requiring renal failure at presentation carries a poor prognosis.
Conclusion
In this study from North India, the majority of anti-GBM disease patients presented late in their clinical course. Dialysis-dependent renal failure portends a poor prognosis. Patients with the double positive disease and those who present with mild disease have a good prognosis with treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033-42.
- [Google Scholar]
- Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66:1535-40.
- [Google Scholar]
- Characteristics and prognosis of Chinese patients with anti-glomerular basement membrane disease. Nephron Clin Pract. 2005;99:c49-55.
- [Google Scholar]
- Anti-glomerular basement disease – An Indian scenario. Indian J Nephrol. 2004;14:182-6.
- [Google Scholar]
- Anti-glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am J Clin Pathol. 2006;125:445-50.
- [Google Scholar]
- Alveolar hemorrhage in anti-basement membrane antibody disease: A series of 28 cases. Medicine (Baltimore). 2007;86:181-93.
- [Google Scholar]
- Anti-glomerular basement membrane disease: Role of enzyme-linked immunosorbent assays in diagnosis. Biochem Mol Med. 1996;59:52-6.
- [Google Scholar]
- Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36:107-13.
- [Google Scholar]
- Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123:1773-83.
- [Google Scholar]
- Predictors of renal and patient outcomes in anti-GBM disease: Clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30:814-21.
- [Google Scholar]
- Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253-62.
- [Google Scholar]







