Translate this page into:
Antibody Response to Covishield and Covaxin in Kidney Transplant Recipients
Corresponding author: Shyam Bihari Bansal, Department of Nephrology and Kidney Transplantation, Medanta Institute of Kidney and Urology, Medanta-The Medicity, Gurgaon, Haryana, India. E-mail: drshyambansal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Manhas N, Bansal SB, Mahapatra AK, Rana A, Sethi SK, Jain M, et al. Antibody Response to Covishield and Covaxin in Kidney Transplant Recipients. Indian J Nephrol. 2025;35:277-82. doi: 10.25259/ijn_549_23
Abstract
Background
The COVID-19 pandemic had a major impact on solid organ transplant recipients. COVID-19 vaccination plays a crucial role in pandemic management.There is limited data on replication-defective viral vectors [ChAdOx1-nCOV (COVISHIELDTM)] and whole inactivated one BBV-152 (COVAXINTM) in kidney transplant recipients (KTRs). This study aims to assess the humoral immune response and adverse effects of these vaccines in KTRs after the first and second doses of vaccination.
Materials and Methods
Anti-SARS-CoV-2 anti-spike antibody titers were measured in 285 KTRs recipients prior to vaccination, 3 weeks ± 3 days after first dose and 3 weeks ± 3 days after second dose of the COVISHIELD (n = 232) and COVAXIN (n = 55) vaccines. Anti-spike antibodies were measured by the chemiluminescence immunoassay method. The primary outcome was seroconversion after two doses of COVAXIN and COVISHIELD and secondary outcome was the incidence of adverse events to COVID-19 vaccines within one week of vaccination.
Results
At baseline, 25 (39.7%) and 67 (30.2%) of KTRs were found to be seropositive before receiving COVAXINTM and COVISHIELDTM, respectively. After first dose of vaccination, 46 (73.0%) and 158 (71.2%) were seropositive and after second dose, 51 (81.0%) and 177 (79.7%) were seropositive, respectively. Common adverse effects were fever, chills, myalgia, and headache which settled in 1–2 days. There was no episode of rejection.
Conclusion
Both ChAdOx1-nCOV and BBV-152 were well tolerated and induced robust antibody formation in KTRs in the Indian population.
Keywords
COVID vaccine
COVAXIN
COVISHIELD
Humoral response
India
Kidney transplant
Seropositivity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread globally, since its first detection in Wuhan China in December 2019, and caused significant morbidity and mortality.1 As per WHO data, SARS-CoV-2 has affected more than 664 million people and caused over 6.7 million deaths worldwide, as of January 25, 2023.2 To curb this, several novel vaccines received an Emergency Use Authorization (EUA) all over the world, including the Indian Central Drugs Standard Control Organization (CDSCO).3
The Indian national vaccination program started on January 16, 2021, after the approval of two candidate vaccines, namely COVISHIELDTM [an adenovirus-vectored vaccine expressing the SARS-CoV-2 spike protein (ChAdOx1-nCOV or AZD1222, acquired from Oxford University and AstraZeneca) manufactured by Serum Institute of India, Pune, India] and COVAXINTM (BBV-152- an inactivated whole virus–based vaccine, manufactured by Bharat Biotech, Hyderabad). As of March 28, 2023, more than 952 million people had completed 2 doses of vaccination.2 However, the data available on the serological response to COVISHIELDTM and COVAXINTM is based on immunocompetent individuals.3
Immunity to SARS-CoV-2 has been shown to provide a good protection against infection and/or reduce morbidity and mortality whether induced through natural infection or via vaccination. Subjects who were seropositive had 89% protection from infection, and vaccine efficacies from 50 to 95% was reported in the general population.4
The pandemic has been particularly deleterious for kidney transplant recipients (KTRs).5,6 To protect these populations, SARS-CoV-2 vaccination is recommended.4,7 However, solid organ transplant recipients were excluded from most initial clinical trials of SARS-CoV-2 vaccines. A low immune response after mRNA COVID-19 vaccine in solid organ transplant recipients has been reported.8-10
In India, COVISHIELD and COVAXIN have been used for the general population and transplant recipients, however, little data are available for response to these vaccines in KTRs.11-13
We assessed the humoral immune response to ChAdOx1-nCOV (COVISHIELDTM) and BBV-152 (COVAXINTM) in and the adverse effects of these vaccines in our kidney transplant recipient population.
Materials and Methods
This prospective study was conducted at Medanta-The Medicity, Gurugram from July 2021 to February 2022. Inclusion criteria were, post-renal transplant recipients more than 18 years of age receiving the COVID-19 vaccine—either COVISHIELD or COVAXIN. Patients were excluded if previously known to be infected with COVID-19 and received plasma, patients who had undergone kidney transplants within 3 months, those with graft rejection within the past 3 months, and thosewith graft failure. Patients who died between the two doses of the COVID-19 vaccine were also excluded.
A total of 318 kidney transplants were screened for eligibility. After excluding 18 patients, 300 were enrolled. Further, 15 patients were excluded from the final analysis due to missing baseline characteristics (n = 9) and follow-up data (n = 6). Detailed clinical history and relevant investigations were recorded. Patients were followed up and the antibody titer was measured at 3 weeks ± 3 days after the first dose and 3 weeks ± 3 days after the second dose of the COVID-19 vaccine. Data regarding any adverse events within a week post-vaccination was also collected.
Baseline antibody titers were measured in all kidney transplant recipients including those who had recovered from SARS-CoV-2 infection in the recent past (>6 weeks before the first dose), Immune response was defined as seropositivity to anti-spike antibody measured by chemiluminescence immunoassay method (CLIA) and chemiluminescent microparticle immunoassay (CMIA). In CLIA, the SARS-CoV-2 S1/S2 IgG antibody concentrations (AU/mL) were measured by the fully automated LIAISON® SARS-CoV-2 S1/S2 analyzer (Dia Sorin S.p.A, USA). The kit was Conformitè Europëenne Mark (CE Mark) equivalent to Bureau of Indian Standard (BIS) certification in India. Antibody levels >15.0 arbitrary unit (AU)/mL were considered as seropositive. For CMIA, anti-spike IgG antibodies to SARS-CoV-2 were assayed with the AdviseDx SARS-CoV-2 IgG II assay (Abbott Diagnostics, Chicago, IL, USA) on the Alinity i system (Abbott Diagnostics, Chicago, IL, USA). The analytical measurement interval is stated as 22–40 000 arbitrary unit (AU)/mL, and the positivity cutoff is ≥50 AU/mL (manufacturer defined). An informed consent was obtained from all patients before their enrolment in the study. IRB approval was obtained with MIRB 1322/2021.
Statistical analysis
The data was not normally distributed. Therefore, non-parametric tests were performed. Descriptive statistics were presented as numbers and percentages. Independent sample Student t-test and Mann-Whitney-U test were used to compare continuous variables between the two groups. Kruskal–Wallis test was used for repeat measure of IgG titers. Chi-square test was used for the comparison between the two attributes. A two-sided p-value < 0.05 was considered statistically significant.
The analysis focused on the change in titers after the first and second doses of the COVID-19 vaccine in relation to baseline titers. All statistical analyses were performed using Statistical Package for Social Science (SPSS software, version 17.0) for Microsoft Windows.
Results
Baseline demographics are listed in Table 1. More than 80% of our study population was ≥50 years of age and were males. Rabbit Anti-thymocyte globulin (ATG- Sanofi) was the most common induction agent used i.e., 37.2% followed by basiliximab in 33.7% and ATG-F (Zydus) in 6.0% patients; 23.2 % of the study population did not receive any induction agent. Most patients (92.6%) received CNI (Tacrolimus), MMF-steroid combination as maintenance immunosuppression [Table 1]. Our study included 13% ABO incompatible kidney transplants, 4 deceased donor transplants, and 8 swap donor kidney transplants.
| Number of patients (n = 285) | Percent (%) | |
|---|---|---|
| Age | ||
| <50 years | 53 | 18.6% |
| ≥50 years | 232 | 81.4% |
| Gender | ||
| Female | 51 | 17.9% |
| Male | 234 | 82.1% |
| BMI | ||
| Underweight | 8 | 2.8% |
| Normal | 193 | 67.7% |
| Overweight | 53 | 18.6% |
| Obese | 31 | 10.9% |
| Years since surgery | ||
| >5 | 74 | 26.0% |
| 3–5 | 73 | 25.6% |
| 2–3 | 72 | 25.3% |
| 1–2 | 66 | 23.2% |
| Induction | ||
| ATG | 106 | 37.2% |
| ATG-F | 17 | 6.0% |
| BASILIXIMAB | 96 | 33.7% |
| No Induction | 66 | 23.2% |
| ABO compatibility | ||
| ABO compatible | 248 | 87.0% |
| ABO incompatible | 37 | 13.0% |
| Maintenance immunosupression | ||
| CNI-antimetabolite-steroid | 264 | 92.6% |
| CNI-steroid | 21 | 7.4% |
| History of recent COVID infection (<6 weeks) | ||
| Yes | 4 | 1.4% |
| No | 281 | 98.6% |
| Baseline SARS CoV IgG titer | ||
| Positive | 92 | 32.3% |
| Negative | 193 | 67.7% |
| Vaccination | ||
| COVAXIN | 63 | 22.1% |
| COVISHIELD | 222 | 77.9% |
BMI: Body mass index, CGN: Chronic glomerulonephritis, CIN: Chronic interstitial nephritis, ATG: Anti-thymocyte globulin, ATG-F: Anti-thymocyte globulin fresenius, CNI: Calcineurin inhibitor.
Majority of our patients (77.9 %) received the COVISHIELD and the rest received COVAXIN (22.1%) [Table 1]. Around 32% of our study population was seropositive prior to vaccination despite having no history or symptom of COVID-19 infection. Of those receiving COVISHIELD, 71.2% became seropositive after the first dose compared to 73.0% who received COVAXIN (p = 0.774) and 79.7% and 81.0% became seropositive after receiving the second dose of COVISHIELD and COVAXIN, respectively [Figure 1, Table 2]. No statistically significant difference was found between the type of vaccination used (p = 0.892).
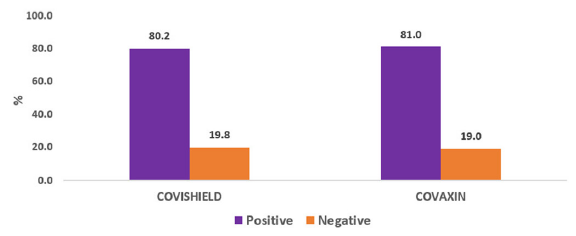
- Total seropositivity and vaccination.
| Vaccination | COVID-19 sero positivity | ||
|---|---|---|---|
| Before first dose of vaccination (baseline) n (%) | After first dose of vaccination n (%) | After second dose of vaccination n (%) | |
| COVAXIN | 25 (39.7%) | 46 (73.0%) | 51 (81.0%) |
| COVISHIELD | 67 (30.2%) | 158 (71.2%) | 177 (79.7%) |
Only 69.4% of our study population who were ≥50 years of age turned seropositive after first dose of COVID vaccination compared to 81.1% who were <50 years of age (p = 0.087) whereas 76.7% vs. 94.3% were seropositive after the second dose of vaccine in ≥50 years of age vs. those <50 years of age (p-value 0.004) [Table 3].
| n = 285 | COVID-19 sero positivity | p-value | |||
|---|---|---|---|---|---|
| Baseline titer (%) (95% CI) | Titer after 3 weeks ± 3 days 1st dose (%) (95% CI) | Titer after 3 weeks ± 3 days of 2nd dose (%) (95% CI) | |||
| Gender | |||||
| Female | 51 | 33.3 (20.8–47.9) | 66.7 (52.1–79.2) | 78.4 (64.7–88.7) | <0.0001* |
| Male | 234 | 32.1 (26.1–38.4) | 72.6 (66.5–78.3) | 80.8 (75.1–85.6) | <0.0001* |
| p = 0.859 | p = 0.391 | p = 0.703 | |||
| Age (years) | |||||
| ≤ 50 | 53 | 35.8 (23.1–50.2) | 81.1 (68.0–90.6) | 94.3 (84.3–98.8) | <0.0001* |
| > 50 | 232 | 31.5 (25.5–37.9) | 69.4 (63.0–75.3) | 77.2 (71.2–82.4) | <0.0001* |
| p = 0.538 | p = 0.087 | p = .005* | |||
| BMI Groups | |||||
| Underweight | 8 | 25.0 (3.2–65.1) | 50.0 (15.7–84.3) | 75.0 (34.9–96.8) | 0.050 |
| Normal | 193 | 33.7 (27.1–40.8) | 72.5 (65.7–78.7) | 81.9 (75.7–87.0) | <0.0001* |
| Overweight | 53 | 30.2 (18.3–44.3) | 69.8 (55.7–81.7) | 79.2 (65.9–89.2) | <0.0001* |
| Obese | 31 | 29.0 (14.2–48.0) | 74.2 (55.4–88.1) | 74.2 (55.4–88.1) | <0.0001* |
| p = 0.891 | p = 0.551 | p = 0.750 | |||
| Induction | |||||
| ATG | 106 | 35.8 (26.8–45.7) | 67.9 (58.2–76.7) | 77.4 (68.2–84.9) | <0.0001* |
| ATG-F | 17 | 35.3 (14.2–61.7) | 82.4 (56.6–96.2) | 88.2 (63.6–98.5) | <0.0001* |
| Basiliximab | 96 | 24.0 (15.8–33.7) | 67.7 (57.4–76.9) | 78.1 (68.5–85.9) | <0.0001* |
| No induction | 66 | 37.9 (26.2–50.7) | 80.3 (68.7–89.1) | 86.4 (75.7–93.6) | <0.0001* |
| p = 0.197 | p = 0.184 | p = 0.379 | |||
| Years since surgery | |||||
| >5 | 74 | 28.4 (18.5–40.1) | 64.9 (52.9–75.6) | 68.9 (57.1–79.2) | <0.0001* |
| 3-5 | 73 | 35.6 (24.7–47.7) | 76.7 (65.4–85.8) | 84.9 (74.6–92.2) | <0.0001* |
| 2-3 | 72 | 30.6 (20.2–42.5) | 69.4 (57.5–79.8) | 84.7 (74.3–92.1) | <0.0001* |
| 1-2 | 66 | 34.8 (23.5–47.6) | 75.8 (63.6–85.5) | 83.3 (72.1–91.4) | <0.0001* |
| p = 0.757 | p = 0.346 | p = 0.040* | |||
| History of recent COVID infection | |||||
| YES | 4 | 50.0 (6.8–93.2) | 75.0 (19.4–99.4) | 100.0 (39.8–100.0) | 0.223 |
| NO | 281 | 32.0 (26.6–37.8) | 71.5 (65.9–76.7) | 80.1 (74.9–84.6) | <0.0001* |
| p = 0.445 | p = 0.879 | p = 0.319 | |||
| ABO incompatible | |||||
| ABO compatible | 248 | 33.9 (28.0–40.1) | 73.0 (67.0–78.4) | 81.9 (76.5–86.4) | <0.0001* |
| ABO incompatible | 37 | 21.6 (9.8–38.2) | 62.2 (44.8–77.5) | 70.3 (53.0–84.1) | <0.0001* |
| p = 0.137 | p = 0.173 | p = 0.098 | |||
| Maintenance immunosuppression protocol | |||||
| CNI-antimetabolite-steroid | 264 | 33.3 (27.7–39.4) | 72.3 (66.5–77.7) | 81.8 (76.6–86.3) | <0.0001* |
| CNI-steroid | 21 | 19.0 (5.4–41.9) | 61.9 (38.4–81.9) | 61.9 (38.4–81.9) | <0.0001* |
| p = 0.178 | p = 0.307 | p = 0.027* | |||
BMI: Body mass index, ATG: Anti-thymocyte globulin, ATG-F: Anti-thymocyte globulin fresenius, CNI: Calcineurin inhibitor, *: statistically significant, CI: Confidence interval.
Sex, BMI, and basic disease did not seem to affect seropositivity after COVID-19 vaccination in our study population. The type of induction was not found to be statistically significant in response to vaccination (p = 0.379) [Table 3].
The median IgG titer in the COVAXIN™ group was 73.6 AU/mL (IQR: 33.9–349.7) by CLIA and 93.3 AU/mL (IQR: 50.7–869.3) by CMIA method and the COVISHIELD™ vaccination group was 188.7 AU/mL (IQR: 32.4–400) by CLIA and 305.9 AU/mL (IQR: 50–2694.3) by CMIA method [Table 4].
| CLIA AU mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline SARS-CoV IgG titer | Titer after 3 weeks ± 3 days of 1st dose | Titer after 3 weeks ± 3 days of 2nd dose | Chi-square value | p-value | ||||||||
| COVISHIELD | 146 | 3.8 (3.8–19.4) | 89.6 (14.9–250.9) | 188.7 (32.4–400) | 239 | <0.0001* | |||||||
| COVAXIN | 45 | 3.8 (3.8–24.4) | 56.2 (16.6–205.7) | 73.6 (33.9–349.7) | 66.7 | <0.0001* | |||||||
| Total | 191 | 37.3 (0.6–58) | 65.4 (28.5–418.7) | 93.3 (50.7–869.3) | 305.6 | <0.0001* | |||||||
| CMIA | |||||||||||||
| n | Baseline SARS-CoV IgG titer | Titer after 3 weeks ± 3 days of 1st dose | Titer after 3 weeks ± 3 days of 2nd dose | Chi-square value | p-value | ||||||||
| COVISHIELD | 74 | 28 (3–57.9) | 99.3 (40.7–670.5) | 305.9 (50–2694.3) | 130.5 | <0.0001* | |||||||
| COVAXIN | 17 | 37.3 (0.6–58) | 65.4 (28.5–418.7) | 93.3 (50.7–869.3) | 28.4 | <0.0001* | |||||||
| Total | 91 | 28.7 (2.5–57.4) | 99.2 (39.1–642.2) | 273 (50.5–1947.2) | 158.8 | <0.0001* | |||||||
CLIA: Chemiluminiscence Immuno assay; CMIA: Chemiluminiscence microparticle assay; *: statistically significant; IQR: Interquartile range; SARS-CoV: Severe acute respiratory syndrome coronavirus
Fever with chills, malaise, and myalgia were the most common side effects after the first dose of the COVID-19 vaccine in our study population. Similar side effects were seen with COVISHIELD and COVAXIN (p = 0.854). Fever with chills followed by malaise was the most common side effect after the second dose of COVID-19 vaccines (p = 0.032). 17.5% of patients who received COVAXIN and 6.8% of patients who received COVISHIELD did not experience any adverse effect after the second dose of vaccination.
Discussion
Vaccination has been crucial in controlling COVID-19. This study documents robust seroconversion after both COVISHIELDTM and COVAXINTM. Not surprisingly, younger patients had better seroconversion. This is comparable to other studies.10,11,13,14 The COVAT study conducted in Indian healthcare workers, showed that people with more than 60 years of age had a significantly lower seropositivity rate.3
This seroconversion rate in this study is higher than that reported with mRNA-based vaccines in kidney transplant recipients from Western population studies but inferior to that seen in the general population.3 In general, COVID-19 vaccines were well tolerated. The safety profile was consistent with the results of previous studies.8,15
Various studies have shown that KTRs elicit an inadequate immune response to the SARS-CoV-2 vaccine. Boyarsky et al. reported that 82.6% of transplant recipients did not mount significant anti-spike antibody titers after the first dose and second dose of mRNA vaccine.8,9 AlShaqaq et al. found that only 23.6% of KTRs developed anti-spike antibodies after one vaccine dose and 35.8% showed a positive response following the second dose.16 Several other studies also reported significantly lower IgG titres in response to the COVID-19 vaccine in kidney transplant recipients.17-19
Most of these studies were done on patients who had received mRNA vaccines, which are not available in India.
In this study, no significant difference was observed in the seroconversion rate of COVISHIELDTM vs. COVAXINTM. Similar observations were made in other studies conducted on KTRs in India.11,13 No statistically significant difference was found between seroconversion of males and females in this study which was similar to other published studies.11,13,16,20
The younger age in these cohorts in our study might explain better seroconversion in response to COVID-19 vaccination compared to a Western population where the median age of patients undergoing renal transplant is higher.8-10 Similar seroconversion and younger age have been seen in other studies conducted in India.11,13 The relatively depressed immune system of older people with the addition of immunosuppressive medications may have resulted in poor immune response to vaccination.21
BMI was found to have an association with seroconversion in response to COVID-19 vaccine.22 No statistically significant difference was found in seroconversion in response to vaccination based on the BMI of post-kidney transplant recipients in this study.
There was no statistically significant difference seen in antibody response to COVID-19 vaccine depending on the type of induction used and ABOc vs ABOi. This was in accordance with other studies.11,13,16
This study adds to the existing evidence on COVID-19 vaccines i.e., COVISHIELD and COVAXIN in KTRs in the Indian population and demonstrates that they are safe and efficacious. This study also has certain limitations. It lacks an immunocompetent control group and it was not possible to investigate cellular immune responses which may play an essential role in protection against COVID infection. There may be a decline in anti-spike antibody titre over time in post-kidney transplant recipients and also long-term side effects of these vaccinations are still unknown.
COVISHIELD and COVAXIN were found to be well tolerated and induced robust antibody formation in kidney transplant recipients in the Indian population. The seroconversion was better in younger patients.
Conflicts of interest
There are no conflicts of interest.
References
- Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant.. 2020;20:3149-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO coronavirus disease (COVID-19) dashboard. Available from https://covid19.who.int/ [Accessed on March 05, 2023]
- Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492-509.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early report on published outcomes in kidney transplant recipients compared to nontransplant patients infected with coronavirus disease 2019. Transplant Proc. 2020;52:2659-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An initial report from the French SOT COVID registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney int. 2020;98:1549-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Is COVID-19 infection more severe in kidney transplant recipients. Am J Transplant. 2021;21:1295-303.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Disponible sur:Available from https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-vaccinationandprioritisation-strategies [Accessed on 2 avr 2021]
- Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. Jama. 2021;325:1784-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. Jama. 2021;325:2204-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17:785-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Seroconversion rate after SARS-CoV-2 infection and two doses of either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ vaccination in renal allograft recipients: An experience of two public and private tertiary care centre. Front Immunol. 2022;13:911738.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibody response to ChAdOx1 nCoV-19 (AZD1222) vaccine in kidney transplant recipients. Vaccines. 2022;10:1693.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Post-vaccination analysis of anti-spike antibody responses in kidney transplant recipients with and without COVID-19 infection in a tertiary care centre, India. Clin Kidney J. 2022;15:1312-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. The Lancet. 2021;397:1819-29.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccination in solid organ transplantation. Am J Transplant. 2013;13:311-7.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and antibody response to BNT162b2 and ChAdOx1 nCoV-19 vaccines in kidney transplant recipients. J. Environ. Sci Pub Health. 2021;5:411-23.
- [Google Scholar]
- Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney int. 2021;99:1487-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody and T cell response to SARSCoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105:e72-3.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of the COVID19 vaccine in kidney transplant recipients. Cureus. 2022;14:e24753.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age-related heterogeneity in neutralising antibody responses to SARS-CoV-2 following BNT162b2 vaccination.
- Influence of obesity on serum levels of SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS One. 2021;16:e0245424.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







