Translate this page into:
Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We evaluated the effects of propolis extract on renal oxidative stress induced by doxorubicin throughout an analytical and pharmacological study of the eastern Algerian propolis using thin layer chromatography, ultra-violet-high-performance liquid chromatography) and gas chromatography-mass spectrometry. The pharmacological study was carried out in vivo on Wistar rat pre-treated with propolis extract 100 mg/kg/day for seven days. Doxorubicin at 10 mg/kg of body weight was administered intravenously on Day 7. Serum creatinine concentration, scavenging effect of flavonoids, lipid peroxidation and glutathione concentration were measured. Chemical analysis allowed identification and quantification of the phenolic compounds including pinostrombin chalcone (38.91%), galangin (18.95%), naringenin (14.27%), tectochrysin (25.09%), methoxychrysin (1.14%) and a prenylated coumarin compound suberosin (1.65%). The total flavonoid concentration in the propolis extract was 370 mg (quercetin equivalents QE) /g dry weight (QE/g DWPE). Propolis extract restored the renal functions and reduced the toxic effect of doxorubicin. These data show a protective effect of Algerian propolis extract against doxorubicin-induced oxidative stress.
Keywords
Algerian propolis
chemical analysis
flavonoids
renal oxidative stress
Introduction
Propolis is a complex resinous hive product, a mixture of wax, sugars and plant exudates collected by bees from plants sources.[1–4] Its chemical and constituents composition depends on its floral origin, and variesaccording to climatic and geographical conditions.[56] Flavonoids and phenolic compounds appear to be the principal components responsible for the biological activities.[6] By their antioxidant activity, flavonoids are able to attenuate the development of cancer and inflammatory diseases.[7] Indeed, one of the most exciting recent findings about propolis is its efficacy in cancer prevention and treatment.[89] Propolis inhibits cancer cell growth by increasing the process of apoptosis by a pro-oxidant effect inducing apoptosis in human melanoma cells or by oxygen species production. In addition, propolis can prevent drug side-effects and reduce drug resistance.
Oxidative stress is now known as the main cause of disturbance in the cellular metabolic oxidant-antioxidant balance.[10] Many situations can cause the production of these oxidants in the body. Several anticancer drugs produce reactive oxygen species (ROS) that may be responsible for the toxicity and harmful adverse effects.[9–12] The administration of doxorubicin leads to the formation of reactive metabolites (e.g, radical's superoxydes O2- and hydrogene peroxides H2O2). The latter attack cell membranes, which leads to the peroxidation of polyunsaturated fatty acids, the release of MDA, and depletion of GSH. Recently, we have shown that the propolis extract protects the liver and the heart tissues against doxorubicin toxicity.[9] The purpose of this research is to investigate the protective effect of the propolis extract against doxorubicin nephrotoxicity and further elucidate whether propolis flavonoids can protect kidney cells by analyzing their scavenging ability and effects on lipid peroxidation and on glutathione reserve.
Materials and Methods
The study was performed on three-month old male albino Wistar rats (Pasteur Institute of Algiers, Algeria) each weighing approximately 250 g. The animals were divided into four groups, with free access to water and food. The ambient temperature was set at 22°C, with a relative humidity of 60% and a light/dark period of 12 h, with the light period beginning at 06:00 am.
The animals were divided into four groups: 1) Eight control rats receiving saline solution 0.9% daily for seven days, 2) Eight rats receiving an oral administration of propolis extract (100 mg/kg/day) for seven days, 3) Five rats receiving an oral administration of propolis extract (100 mg/kg/day) for seven days before the intravenous injection of 10 mg/kg doxorubicin on the seventh day, and 4) Five rats receiving a single intravenous injection of 10 mg/kg doxorubicin alone.
The blood was collected at 24 h, seven and 14 days from the retro-orbital sinus in tubes and centrifuged at 3300 rpm for 10 min. The serum was collected and frozen for biochemical and enzymatic analyses. The urine was collected for measuring the activity of λ-glutamyl transpeptidase at 24 hours, seven days and 14 days. The rats were sacrificed after times 24 hours, seven days and 14 days after administration of the anticancer drug doxorubicin. Kidneys were removed and frozen (–20°C) until assay.
Crude propolis (beeswax and resins collected by the honeybee from plants) was obtained from beekeepers of the “Cooperative Apicol of Kaous, Jijel (Eastern Algeria)” in May 2008. Samples, once received, were stored at 4°C in airtight /dark plastic containers until analysis.
The bioactive substances of propolis were extracted by ethanol and methanol. One hundred grams of raw propolis were cut in small pieces, added with nine volumes (900 ml) of 95% ethanol, the mixture was let for steeping for 15 days with agitation. After filtration on cotton, the filtrate was evaporated at 80°C using a rotary evaporator (Evaporator E100, Heidolph-Instruments, Germany). The residual was retaken using 70% methanol and was let for steeping overnight. After evaporation of the solvent, the raw extract or ethanolic propolis extract (EEP) was obtained. The extraction was continued by passage in various solvents to obtain aglycones and heterosides flavonic. Flavonoοds rate was determined by reactivity with aluminium chloride (AlCl3) as described by Benguedouar et al.[8]
Thin-layer chromatography (TLC) was performed on silica gel plates 60-GF254 (Merck). The EEP samples were diluted with 95% ethanol (1/10 vol/vol) and 5 μl of the propolis solution were applied to the plates. The mobile phase used was pure chloroform. The TLC chamber was saturated with the mobile phase at least 1h before analysis. After developing, plates were dried in the air, and propolis components were visualized under ultraviolet light (336 nm) after spraying with 1% ethanolic solutions of aluminum chloride. The TLC of EEP was used to identify the components of propolis.
Analyses of flavonoids and other phenolic acids was carried out using reversed phase high-performance liquid chromatography–diode array detection (RP-HPLC-DAD). The HPLC system was from Varian; DAD was a Varian Prostar monitoring from 200 to 700 nm wavelengths (Middelburg, The Netherlands). The phenolic compounds in EEP at concentration of 10 mg/ml were separated on a Nucleodur analytical column, 250 mm × 4.6 mm i.d (Machery-Nagel, Duren, Germany) packed with C18 stationary phase, particle size 5 mm.
The linear binary gradient was used. The time of the HPLC run was over 70 min. The binary mobile phase consisted of Solvent A (trifluoroacetic acid 0.01%) and Solvent B (acetonitrile). The separation was obtained by using a gradient starting at 10% of B till 10 min; 10-50 min B increased to 50% and kept constant till 15 min; 65-70 min B increased at 100% and kept constant till 10 min; successively, Solvent B reached back 10% to reequilibrated the column (washing).
Gas chromatography-mass spectrometry (GC-MS) was carried out on a Fisons GC 2010 gas chromatograph coupled to a Fisons MSQP_2010 mass detector under electron impact ionization (70 eV) at a maximum temperature of 350°C. The chromatographic column for the analysis was non-polar (FS-FE 30 CB) capillary column (25 m ×0.25 mm i.d). The carrier gas used was helium, and the sample volumes (0.5 μl) of EEP solutions were injected. Two programs were tested, the first in EEP, with the initial temperature 150°C, the final temperature 280°C and a stage 10°C/min, the second in different fractions (i.e., the phase ether diethyl or aglycone flavonic), with the initial temperature 100°C, the final temperature 280°C and a stage 3°C/min.
The free radical scavenging activity of test compounds (propolis extract, and quercetin) was evaluated using the stable radical 1, 1-diphenyl-2-picrylhydrazyl (DPPH°). 15 μl of each compound's test was mixed with 1.5 ml (1mg) DPPH° in ethanol. The absorbance of the remaining DPPH° was determined every 15 sec for 5 min at 515 nm. Blank sample contained the same amount of ethanol and DPPH°. The measurements were performed in triplicate. The radical scavenging activity was calculated by the formula: I= [(Aa – Ab] *100; where
I = DPPH° inhibition,;
Ab = absorption of blank sample (t = 0 min);
Aa = absorption of tested compounds.
Homogenates of the cortical and outer medullar zones of the kidney were prepared at 10% (w/v) in 0.1 mol/L Tris-HCl buffer, pH 7.4, and malondialdehyde (MDA) steady-state levels were determined. MDA was measured according to the method described by Sastre et al.[13] Thiobarbituric acid 0.67% (w/v) was added to aliquots of the homogenate previously precipitated with 10% trichloroacetic acid (w/v). Then the mixture was centrifuged, and the supernatant was heated (100°C) for 15 min in a boiling water bath. After cooling, n-butanol was added to neutralize the mixture, and the absorbance was measured at 532 nm. The results were expressed as nmol of MDA/g tissue.
Cellular glutathione content was measured as described by Ellman.[14] 0.5g of fresh kidney tissue was homogenized with three volumes of trichloroacetic acid (TCA) (5%) using a grinder DOUNCE. After centrifugation at 2000 rpm, 50μl of the supernatant was diluted in 10 ml of phosphate buffer (0.1M, pH = 8). 20 μl of DTNB 0.01 M (acid 5, 5’- dithiobis 2-nitrobenzoic acid) was added to 3 ml of the mixture dilution. The measurement of the optical density was performed at 412 nm against a control prepared in the same conditions using TCA 5%. The concentrations are expressed in mmoles of glutathione /g of kidney.
Serum creatinine was measured by the Jaffe's method.[15] The enzymatic activity of GGT in urine was measured in 0.1 mol/L Tris-HCl buffer (1M Tris, 100 mM MgCl2), pH 9.0, following the method of Beck and Thomson[16]. Fifty μl were incubated at 25°C in the presence of 5 mmol/L L-gamma-glutamyl-p-nitroanilide. The enzymatic reaction was stopped by the addition of 1 ml of 1N acetic acid and the rate of p-nitroanilide release was determined spectrophotometrically at 405 nm. One unit of GGT activity was defined as the amount of enzyme releasing μmol of p-nitroanilide per minute. The specific activity of the enzyme was expressed as units/mg protein.
Data are presented as mean and standard deviation. The averages of the groups treated with drugs were compared with those of control and flavonoid-treated groups using one-way analysis of variance (ANOVA) and t-test. P-values less than 0.05 were considered significant.
Results
The different phenolic compounds were identified by their UV spectra, recorded with a DAD coupled to the HPLC, bathochromic movement of Band I (320-380 nm) and Band II (240-280 nm). HPLC-UV chromatograms of the phenolic fractions of propolis indicate the presence of three classes of polyphenols: flavonoids (maximal absorption at 280 and 370 nm); chalcones: dihydrochalcone (λmax = 280-290 nm) and phenolic acids.
The identification was accomplished using computer searches on commercial libraries [Figure 1]. The components of EEP were determined by considering their areas percentage of the total ion current [Table 1]. The major flavonoids detected in the propolis extract were pinostrobin chalcone, pinocembrin, tectechrysin, chrysin and naringinin.

- Gas chromatography chromatogram of ethanolic extract of propolis (EEP)

One of the more prominent properties of the flavonoids is their excellent radical scavenging ability. The scavenging activity of the propolis extract was observed at concentrations up to 0.12 mg/ml showing it to be stronger than that of the positive control, quercetin [Figure 2]. Indeed, at 1 mg/ml the propolis extract had a percentage reduction of 88.30 against 75.51% for quercetin.
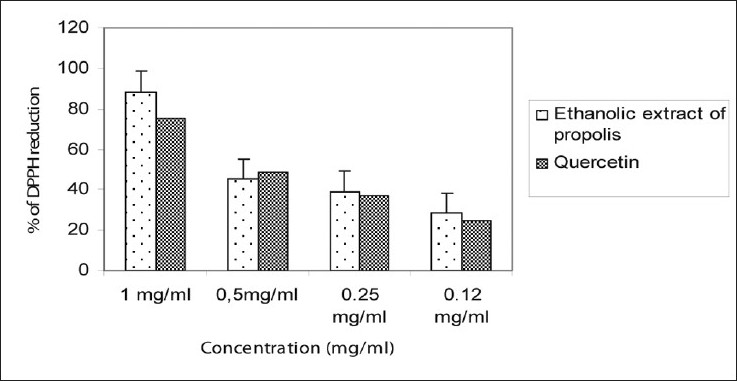
- Scavenger effects of the ethanolic extract of propolis vs quercetin
The propolis extract showed a strong effect on lipid peroxidation [Figure 3]. A significant reduction of malondialdehyde (MDA) concentrations was observed in kidney cells of animals pre-treated with 100 mg/kg propolis extract daily for seven days prior to doxorubicin 10 mg/kg IV injection (25.30±1.13 nmol/g tissue against 75.4±0.96 nmol/g tissue in rats treated with the drug only on day seven). The propolis extract provide protection against free radicals formed during the metabolism of doxorubicin.

- Changes in malondialdehyde levels of renal tissue after a single dose of doxorubicin 10 mg/kg alone or combined with the propolis extract 100 mg/kg. The results are expressed as mean ± SEM
Rats treated with doxorubicin show a very significant (P<0.01) decrease in renal GSH concentrations at day 14 (0.77±0.55 mM/g tissue against 2.97±0.32 mM/g tissue for controls) [Figure 4].
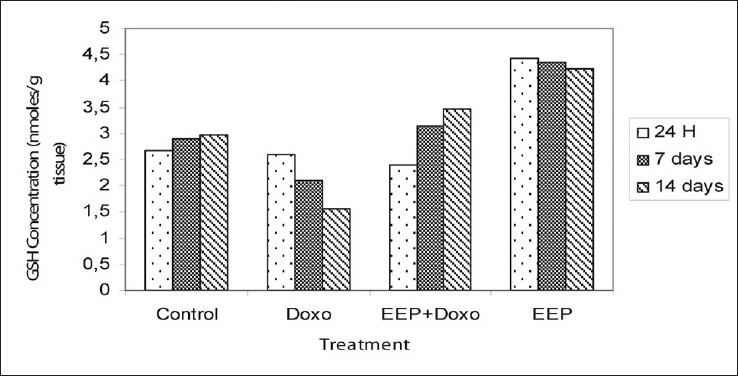
- Changes in rates of renal tissue glutathione after administration of a single dose of doxorubicin 10 mg/kg alone or combined with the propolis extract 100 mg/kg. The results are expressed as mean ± SEM
The rats pretreated with propolis extract showed a significant increase of GSH levels compared to those receiving doxorubicin alone (3.47±0.07 mM against 1.55±0.09 mM) at 14 days. The glutathione concentrations decreased in rats receiving doxorubicin alone compared to that of controls (0.77±0.55 mM) and increased in animals treated with propolis extract (3.47±0.07mM/g tissue).
The concentrations of serum creatinine in rats treated with doxorubicin 10 mg/kg alone or combined with the ethanolic extract of propolis administered for seven days at 100 mg/kg are shown in Figure 5. In rats treated with doxorubicin 10 mg/kg alone, there was a significant increase in plasma creatinine. Twenty-four hours after injection, the creatinine concentration reached 53.69±15.15 μM/L in treated rats against 40.14±8.14 μM/L in controls and increased progressively until day 7 (P<0.001). However, the combination of doxorubicin with propolis extract showed a significant attenuation in serum creatinine (46.8±10.4 μM/L against 63.72±7.21 in rats treated with doxorubicin alone).
- Changes in rates of serum creatinine during treatment with doxorubicin 10 mg/kg alone or combined with the propolis extract 100 mg/kg. The results are expressed as mean ± SEM
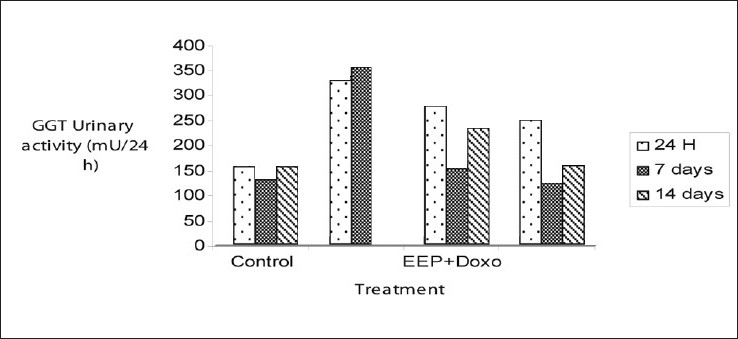
The doxorubicin treated rats showed a highly significant increase of the urinary enzyme activity from the first to seventh day (P<0.001). This activity was increased compared to controls (351.67±7.32 mU against 127.38±2.89 mU) [Figure 6]. However, this was abrogated in rats treated with the ethanolic extract of propolis at 100 mg/kg for seven days followed by a single dose of doxorubicin 10 mg/kg (149.24±3.47 mU/24 h).

- Variations of urinary enzymatic activity of gamma-glutamyltranspeptidase after treatment with doxorubicin 10 mg/kg alone or combined with the propolis extract 100 mg/kg. The results are expressed as mean ± SEM
Discussion
Propolis has many anti-oxidant and antiapoptotic properties. Phenolic compounds (flavonoids and phenolic acid derivatives) are the most important pharmacologically active constituents in propolis. However, the constituents of propolis vary widely with climate and location. The major flavonoids detected from the extracts in our study were, pinostrobin chalcone, tectochrysin. We observed some similarities in the qualitative composition between the Algerian and Turkish propolis.[34] The phenolic compound were similar to those identified in the propolis from American countries[5] and the Mediterranean region.[17] The biological activity of propolis samples needs different compounds combinations.[18–20] Considerable interest in these compounds extracted from propolis has arisen with the recognition for their antibacterial and anticancer effects and also of their antioxidant effects.[102122]
Impairment of renal function was identified in this study by showing elevated serum creatinine and urea concentrations along with proteinuria and enzymuria. The increase in urine gamma-glutamyl transpeptidase (γGT) was used as a sensitive indicator of damage to proximal renal tubule brush borders.[23] These results were consistent with those of Lahouel[24] and Viotte et al.[25] and are also consistent with those of Abo-Salem et al.[26] showing that injection of doxorubicin 10 mg/kg in rats increased plasma creatinine. However, we observed a decrease in plasma creatinine in animals receiving the propolis extract before drug treatment.
Recently, we have reported that flavonoids of propolis extract protect the liver and heart tissues in rats treated with anticancer drugs[9] and we hypothesized that the protective effect of flavonoids could be due to their capacity to capture and deactivate the free radicals. Adopting the method of DPPH (1.1, 2-Diphenyl hydrazyl), our study shows that the ethanolic extract of propolis has a very strong scavenger effect; it is more active at concentrations up to 0.12 mg/ml (reduction of 88.30% against 75.51% with quercetin at concentration 1 mg/ml). Lipid peroxidation is one of the major effects of the radical attack leading to the formation of (MDA).[2728] Our study performed on the kidney showed a significant increase of MDA in groups of animals receiving doxorubicin alone; indicating drug toxicity. At the same time, the rates of glutathione recorded in rats treated with doxorubicin alone were lower than in control rats. This decrease was probably due to the toxic effect of the reactive metabolites of the drug that can be fixed and neutralized by the detoxification system, resulting in decreased GSH concentrations, or degradation the GSH by the gamma-glutamyl transpeptidase. We observed a decrease of MDA in groups of animals receiving the flavonoids before doxorubicin treatment, and increased glutathione levels in groups of animals pretreated with flavonoids of propolis. Taken together these data may explain the preventive effect of flavonoids against renal oxidative stress induced by doxorubicin.[2930] The mechanism by which the natural product propolis extract prevents renal oxidative stress may include an increasing rate of GSH or by induction of its synthesis or by a scavenger effect. Instead of the toxic reactive metabolites binding to glutathione and consume, they will be captured by the flavonoids (naringenin, pinostrombin and galangin).
Abbreviation list
DWPE, dry weight of propolis extract; extract; QE, quercetin equivalents; RP-HPLC-DAD, reversed phase high performance liquid chromatography-diode array detection; UV-HPLC, ultra violet high phase liquid chromatography.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Chemical composition of European propolis: Expected and unexpected Results. N Naturforsch. 2002;57c:530-3.
- [Google Scholar]
- Recent progress in pharmacological research of propolis. Phytoth Res. 2001;15:561-71.
- [Google Scholar]
- CG-MS analysis of propolis samples from two different regions of Turkey. Z Naturforsch. 2002;57c:905-9.
- [Google Scholar]
- Propolis from the Mediterranean region:chemical composition and antimicrobial activity. Z Naturforsch. 2000;55:790-803.
- [Google Scholar]
- Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res. 2008;22:1256.
- [Google Scholar]
- Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta. Pharm. 2004;54:65-72.
- [Google Scholar]
- A study of chemical composition and antimicrobial activity of Bulgarian propolis. Folia. Med (Plovdiv). 2007;49:63-9.
- [Google Scholar]
- Efficiency of propolis extract against mitochondrial stress induced by antineoplasic agents (doxorubicin and vinblastin) in rats. Indian J Exp Biol. 2008;21:112-9.
- [Google Scholar]
- Cardioprotective effects and mechanism of action of Polyphenols extracted from Propolis against Doxorubicin toxicity. Pak J Pharm Sci. 2008;21:201-9.
- [Google Scholar]
- Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food. Chem Toxicol 2008:3140-5.
- [Google Scholar]
- Etude pharmacochimique de l’extrait de propolis au cours d’un stress oxydatif rénal induit par la doxorubicine, Magister de pharmacochimie, université de Jijel. 2007
- [Google Scholar]
- On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem. 1945;158:581-600.
- [Google Scholar]
- Aminoglycoside antibiotics and renal function: Changes in urinary gamma-glutamyltransferase excretion. JClinPathol. 1977;30:432-7.
- [Google Scholar]
- Egyptian Propolis: 2.Chemical Composition, Antiviral and antimicrobial activities of East Nile delta propolis. Z. Naturforsch. 2002;57c:386-94.
- [Google Scholar]
- Synergistic effects of ferritin and propolis in modulation of beryllium induced toxicogenic alterations. Food. Chem Toxicol. 2008;46:3069-79.
- [Google Scholar]
- Plant origin of Okinawan propolis: Honeybee behavior observation and phytochemical analysis. Naturwissenschaften. 2008;95:781-6.
- [Google Scholar]
- Functional properties of honey, propolis, and royal jelly. JFood Sci. 2008;73:R117-24.
- [Google Scholar]
- Effects of propolis and CAPE on proliferation and apoptosis of McCoy-Plovdiv cell line. Folia Med (Plovdiv). 2008;50:53-9.
- [Google Scholar]
- Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia-reperfusion both in vivo and in vitro. Brain Res. 2008;24:104-15.
- [Google Scholar]
- Angiotensin I converting enzyme activity in uranyl nitrate induced acute renal failure in rats. Ren Fail. 1995;17:377-88.
- [Google Scholar]
- Etude de la toxicité hématologique, hépatique et rénale de deux médicaments anticancéreux; la doxorubicine et le CCNU chez le rat. Thèse de doctorat de l’université de Haute Normandie (France) 1985:220.
- [Google Scholar]
- CCNU -Adriamycin association induces earlier and more server nephropathy in rats. Arch Toxicol. 1988;61:282-91.
- [Google Scholar]
- Experimental diabetic nephropathy can be prevented by propolis: Effect on metabolic disturbances and renal oxidative parameters. Pak J Pharm Sci. 2009;2:205-10.
- [Google Scholar]
- Propolis reverses acetaminophen induced acute hepatorenal alterations: A biochemical and histopathological approach. Arch Pharm Res. 2008;31:451-61.
- [Google Scholar]
- Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. BioorgMedChem. 2008;15:5434-40.
- [Google Scholar]
- Recent advances in protection against doxorubicin-induced toxicity. Technol Cancer Res Treat. 2008;7:497-516.
- [Google Scholar]
- Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60 (OH)24 via suppression of oxidative stress. Pharmacol Rep. 2008;60:742-9.
- [Google Scholar]







