Translate this page into:
Plasma cell-rich acute rejection of the renal allograft: A distinctive morphologic form of acute rejection?
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study was aimed at evaluating the clinicopathologic features of plasma cell-rich acute rejection (PCAR) of renal allograft and comparing them with acute cellular rejection (ACR), non-plasma cell-rich type. During a 2-year period, eight renal allograft biopsies were diagnosed as PCAR (plasma cells >10% of interstitial infiltrate). For comparison, 14 biopsies with ACR were included in the study. Detailed pretransplant data, serum creatinine at presentation, and other clinical features of all these cases were noted. Renal biopsy slides were reviewed and relevant immunohistochemistry performed for characterization of plasma cell infiltrate. The age range and duration of transplantation to diagnosis of acute rejection were comparable in both the groups. Histologically, the proportion of interstitial plasma cells, mean interstitial inflammation, and tubulitis score were higher in the PCAR group compared with cases with ACR. A significant difference was found in the outcome at last follow-up, being worse in patients with PCAR. This study shows that PCAR portends a poor outcome compared with ACR, with comparable Banff grade of rejection. Due to its rarity and recent description, nephrologists and renal pathologists need to be aware of this entity.
Keywords
Acute rejection
outcome
plasma cell-rich
renal allograft
Introduction
Plasma cell-rich acute rejection (PCAR) is a morphologic type of acute rejection with prominence of plasma cells in the interstitial infiltrate.[1] There are few studies on patients with PCAR in the available literature.[1–4] The clinical significance and distinction of PCAR from acute cellular rejection (ACR) has been dealt with in only four studies so far.[1–4] In the previous studies, the response to antirejection therapy in PCAR has been less than satisfactory with poor graft survival rates.[1]
The histologic diagnosis of PCAR with prominence of plasma cells mandates consideration of posttransplant lymphoproliferative disorder (PTLD), viral infections, and drug toxicities. Of these, PTLD is the most important differential, which can be differentiated from PCAR by presence of destructive lesions, monoclonality, and demonstration of Epstein-Barr virus within the neoplastic cells of PTLD.[5]
Due to the rarity of PCAR, its inclusion in the Banff classification of renal allograft pathology is still awaited. Recognition of this entity and description of more cases in the literature would help in delineating the clinical features and appropriate therapeutic approach.
This manuscript reports the clinicopathologic features of eight cases of PCAR diagnosed at our institution over a 2-year period.
Materials and Methods
All renal allograft biopsies reported as ACR with plasma cell-rich infiltrate (plasma cells constituting >10% of the infiltrating cells) during the period January 2008 to December 2009 were retrieved. Over this period, eight such cases were identified. For comparison, cases of ACR matched for age, duration of transplant, and Banff score were randomly selected and included in the study.
Clinical information collected from the files of these patients included age, deceased donor versus live-related donor graft, duration of allograft to biopsy diagnosis of acute rejection, extent of HLA matching, level of panel reactive antibodies (PRA), baseline serum creatinine and creatinine at the time of biopsy, serum levels of calcineurin inhibitors (cyclosporine or tacrolimus), therapy given after diagnosis of acute rejection, and subsequent follow-up data. The standard immunosuppressive regime followed at our institution constitutes steroid-based therapy with calcineurin inhibitors (cyclosporine or tacrolimus) along with azathioprine or mycophenolate mofetil. For management of ACR, intravenous methylprednisolone pulse therapy is used.
Serum titers for cytomegalovirus (CMV) and BK virus (BKV) by real-time polymerase chain reaction (RT-PCR) were also estimated in these cases.
All renal allograft biopsies were formalin-fixed and paraffin-embedded for routine light microscopic studies. Serial sections were stained with hematoxylin and eosin, periodic acid Schiff, and methenamine silver stains. The rejection and nonrejection pathology was studied and graded using the 2007 update of Banff classification of renal allograft pathology.[6]
In all cases of PCAR, immunohistochemistry for CD20 (B-cell marker), CD3 (T-cell marker), and kappa and lambda light chains (to detect monoclonality) was performed using the streptavidin-biotin-peroxidase method with 3′,3′-diaminobenzidine as chromogen. In addition, all the cases were stained for C4d (Abcam Inc., Cambridge, USA), CMV (Novocastra Laboratories Ltd, Newcastle upon Tyne, UK), and BKV (BD Biosciences, San Jose, CA, USA). Positivity for C4d was assessed in the peritubular capillaries and scored according to Banff 2007 criteria.[6] Nuclear staining was considered as positive for CMV and BKV.
Results
In the study duration, 254 renal allograft biopsies were received in our department. Of these, eight biopsies (3.14%) from eight patients showed features of PCAR. Fourteen biopsies with ACR without plasma cell prominence were also included for comparison.
Clinical data
There was no significant difference between PCAR and ACR in terms of patient age (34 ± 13 vs. 36 ± 14 years, respectively, P>0.05) and posttransplantation duration (31.9 ± 17.2 vs. 22.5 ± 12.6 months, respectively, P>0.05). The number of females in the PCAR group was marginally significantly higher than the ACR group (male: female 5:3 PCAR vs. 14:0 ACR, P value 0.03). No significant difference was noted between the two groups with respect to the degree of HLA mismatched antigens (2.6 ± 0.5 PCAR vs. 2.5 ± 1.2 ACR, P>0.05), level of PRA, and type of calcineurin inhibitor being received. Serum creatinine at the time of biopsy diagnosis of acute rejection was also comparable in both groups. Serum trough levels of calcineurin inhibitors were similar in both ACR and PCAR groups. The dosage of steroid used and the frequency of use of MMF or azathioprine were also comparable. The salient features of comparison between PCAR and ACR are tabulated in Table 1. None of the patients had received a prior renal allograft. The clinical characteristics and relevant biochemical parameters of patients with PCAR are tabulated in Table 2.


All the patients with PCAR and ACR had received live-related donor grafts and were on steroid-based triple drug immunosuppression. There was no history of noncompliance to immunosuppressive drugs in any of the patients in both the groups. CMV and BKV were negative by real time PCR in all cases.
Histopathology
According to the Banff 2007 criteria, the acute rejection was graded as IA in one case, IB in four, IIB in two, and III in one biopsy of PCAR [Figure 1a–d]. In the ACR group, acute rejection was Banff grade IA in five, IB in six, IIB in one, and III in two biopsies. No significant difference was noted in the grade of rejection between the two groups. The mean interstitial inflammation score was 2.25 (±0.46) in PCAR and 2.18 (±0.4) in ACR group, while the tubulitis score was 2.37 (±0.51) in PCAR and 2.18 (±0.4) in ACR group. On statistical analysis, the difference in the interstitial and tubulitis score was significant (P values 0.01 and 0.03, respectively). Significant interstitial edema was noted in three biopsies with PCAR [Figure 1a]. Chronic tubulointerstitial changes of varying grades (Banff grade I to II) were observed in all biopsies with PCAR and this was comparable with the ACR group.
Plasma cells constituted >10% of the interstitial infiltrating cells in all eight biopsies of PCAR [Figure 1b and c]. The plasma cells were seen to infiltrate in nonfibrotic areas of the cortical parenchyma [Figure 2a]. In two cases, plasma cells were also observed in the foci of tubulitis [Figure 2b]. The infiltrating plasma cells were cytologically mature without nuclear atypia. Immunohistochemistry revealed the plasma cells to be polyclonal for kappa and lambda light chains in all cases [Figure 3a and b]. No significant lymphoid nodule formation, CD20-positive aggregates, or tissue-destructive lesions were noted in any of the biopsies with PCAR. In contrast, the plasma cells were infrequent in the biopsies with ACR. None of the cases showed features suggestive of transplant glomerulopathy.
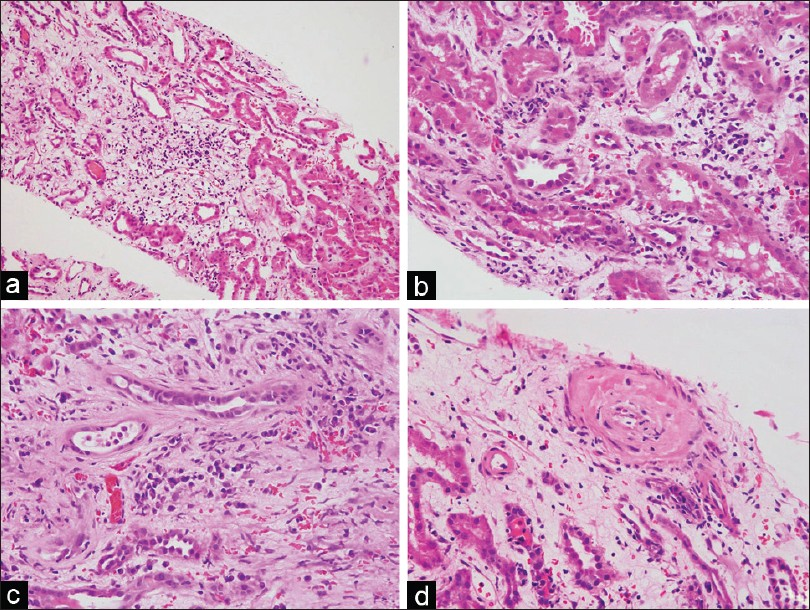
- Photomicrographs from a case of PCAR showing prominent interstitial edema (a, H and E, ×40) and an interstitial infiltrate (b, H and E, ×100) with numerous plasma cells (c, H and E, ×100). Intimal arteritis with reduction of vascular lumen is seen in the same biopsy (d, H and E, ×100)
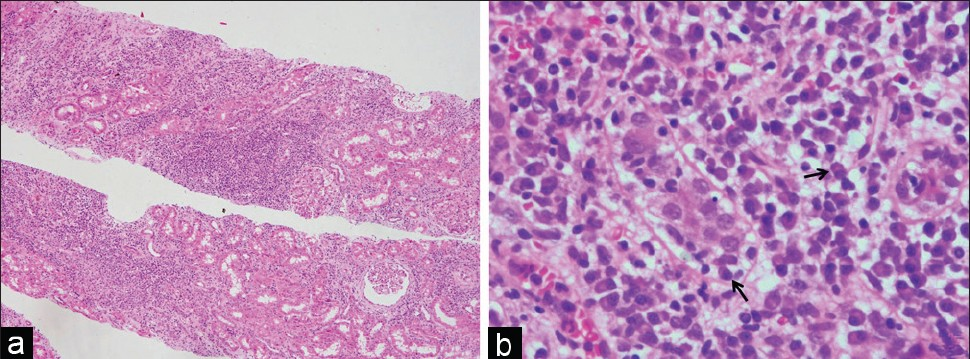
- Photomicrographs from a case of PCAR showing diffuse (i3) interstitial infiltrate (a, H and E, ×40). The interstitial infiltrate demonstrates predominance of plasma cells with occasional plasma cells in the focus of tubulitis (arrows, b, H and E, ×400)
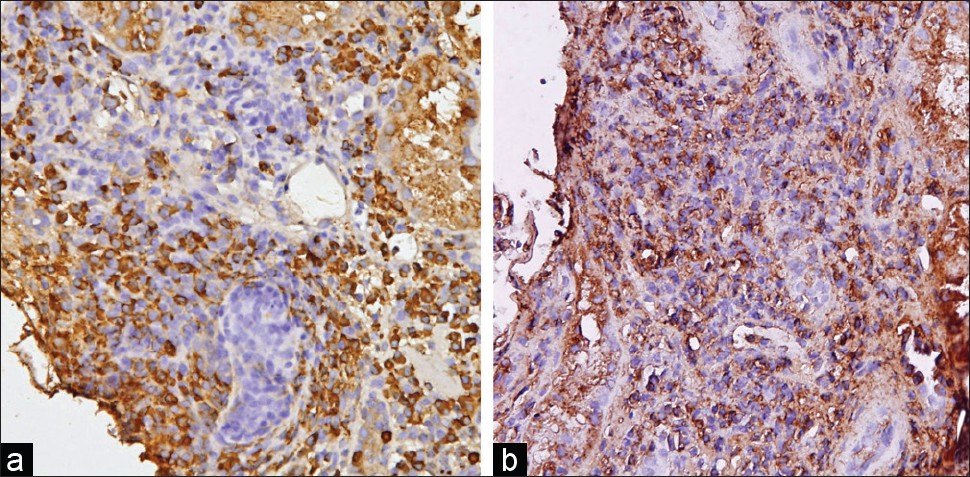
- Immunohistochemistry in a biopsy with PCAR shows an admixture of kappa (a) and lambda (b) positive plasma cells (×200)
C4d staining was performed in all the biopsies included in this study and was found to be negative, with appropriate controls being positive. Immunohistochemical staining for CMV and BKV was also negative in all the cases.
Follow-up
The median duration of follow-up in cases with PCAR was 11 months (range 7–37 months) compared with 12 months (range 8–28 months) in ACR group. Following the biopsy diagnosis of PCAR, seven patients were administered intravenous methylprednisolone pulse therapy while one patient could not be given pulse steroids due to intercurrent sepsis. Of these seven patients, one had good response to antirejection therapy, four had partial response with lowering of serum creatinine, and two had no significant change in creatinine value. Adequate follow-up was available in all patients with PCAR. Of the eight patients, three had graft failure at 7, 17, and 27 months after the biopsy. In four patients, serum creatinine remained high (1.7, 1.8, 2.6, and 3.6 mg/dl) at the last follow-up (13, 13, 6, and 37 months, respectively) while one patient had a serum creatinine of 1.4 mg/dl at 14-month follow-up visit.
In contrast to the ACR group, the outcome (measured in terms of functioning or nonfunctioning grafts) in patients with PCAR was found to be unfavorable. All the patients with ACR received methylprednisolone pulse therapy and had a functioning graft at 8- to 28-month postbiopsy follow-up. Statistical analysis showed a poorer outcome in the PCAR group (P=0.03) compared with the patients with ACR.
Discussion
ACR of the renal allograft usually shows interstitial infiltrate of activated lymphocytes of T-cell origin along with scattered plasma cells, eosinophils, and neutrophils.[1] PCAR is a relatively newly described entity characterized by the presence of plasma cells constituting more than 10% of the infiltrating cell population.[1] There are very few reports/studies of PCAR in the available English literature.[1–47–9] Various studies have shown an incidence of 1.8–2.5% of the total number of allograft biopsies.[127] In this study, PCAR (defined as cellular rejection with >10% plasma cells in the infiltrating population) constituted 3.14% of all allograft biopsies performed at our institution over a 2-year period.
Some studies have compared PCAR with ACR, nonplasma cell-rich.[2–4] Similar to these previous studies, we found a significantly higher proportion of female patients in the PCAR group. However, none of the other clinical parameters, including age, number of pretransplant HLA-mismatched antigens, PRA, or duration of transplant prior to diagnosis of rejection, was significantly different between the PCAR and ACR groups.
The clinical significance of PCAR has been addressed in only four previous studies.[1–4] In these studies, PCAR was found to be resistant to intensified steroid pulse therapy with some response to antithymocyte globulins or OKT3.[12] Occasional cases responsive to intravenous immunoglobulins with or without plasmapharesis respond.[9] In this study, only partial response to intravenous steroid pulse therapy was observed in five patients, while there was no response in the other two patients (one patient could not be administered pulse therapy). In comparison, all the patients in the ACR group responded to pulse steroid therapy. This confirms the earlier observations of a poor response of PCAR to augmented immunosuppression. However, this study was limited by the lack of use of second-line agents such as OKT3 or antithymocyte globulin. The poor response to standard antirejection therapy, including antithymocyte immunoglobulins, in patients with PCAR, has been ascribed to a possibility of antibody-mediated rejection in these patients. In the study by Desvaux et al., 67% of the patients had circulating antibodies, potentially reactive to donor antigens. C4d deposition in the peritubular capillaries was detected in three of five samples stained by immunofluorescence in their study. The authors concluded that antibody-mediated mechanisms were involved in PCAR.[1] However, the characteristic histologic changes of antibody-mediated rejection were not observed in any of their cases. This was explained on the basis of high expression of interferon-gamma in the biopsies with PCAR, as interferon-gamma prevents microvessel injury and necrosis at the microcirculation level.[1] Another drug that has been tried in refractory acute antibody-mediated rejection is proteasome inhibitors (such as bortezomib) which have been shown to deplete transformed and nontransformed plasma cells.[10] In this study, none of the cases showed positive staining for C4d by immunohistochemistry. However, the number of cases in our study is not large enough to draw a conclusive opinion on this subject of the component of antibody-mediated rejection in these patients. In addition, we performed C4d staining by immunohistochemistry. Earlier studies have demonstrated that immunofluorescence is more sensitive for C4d staining.[11] Alternative therapy with proteasome inhibitors could not be tried in any of our cases due to financial constraints.
The graft survival at 6 months after diagnosis of PCAR have been around 50%, much lower than graft survival in non-PCAR rejections matched for Banff scoring. In the study by Desvaux et al., complete response to enhanced immunosuppression was reported in only 28% of the cases, which was lower than in grade IA acute rejections.[112] These data suggest that the presence of plasma cell-rich infiltrates indicates a poor outcome, irrespective of the Banff scoring. In this study, three of eight patients with PCAR had graft failure with return to dialysis compared with none in the ACR group. This difference was statistically significant (P value 0.03). Hence, the results from this study reiterate the poor outcome in PCAR, as reported in other studies.[13]
Plasmacytic infiltrates in the renal graft may also be seen in posttransplant lymphoproliferative disorder (PTLD), viral infections, and exposure to toxins or drugs. PTLD has been subdivided into early (plasmacytic hyperplasia), polymorphic PTLD, and monomorphic B-cell and T-cell lymphomas including plasmacytoma-like lesions and plasma cell myeloma.[13] Polymorphic PTLDs are seen as destructive lesions with architectural effacement in tissue biopsies and high expression of EBV RNA in the infiltrating cells.[514] In this study, none of the cases exhibited monoclonality or expansile destructive lesions in the renal allograft biopsies. The presence of EBV-RNA could not be investigated in our cases. Viral infections of the graft, especially polyoma virus (BKV), are also associated with plasma cell-rich infiltrates. The most important histomorphological feature for the diagnosis of polyoma virus nephropathy is cytopathic effects, including intranuclear inclusions, smudging of nuclear chromatin, and denudation of tubular epithelial cells predominantly affecting the distal nephron segments in the medulla.[15] None of the biopsies in our study showed these histologic features of viral infection.
There are certain limitations of our study, including the lack of protocol biopsies, which allows the renal pathologists to detect subclinical alterations in the graft. In addition the plasma cells were identified and quantified on morphologic basis only, due to the lack of facilities for immunohistochemistry for CD138 or CD38. None of our patients of PCAR could be managed with second-line immunosuppressive therapy due to financial constraints, and hence, the true outcome is not known.
Hence, this study reports eight cases of PCAR, a rare morphologic type of ACR of renal allograft. This subtype of ACR portends a poor response to standard antirejection therapy and worse graft outcome when compared with cases with a comparable Banff grade of ACR. Hence, early accurate recognition of this morphology in a renal allograft biopsy is imperative for appropriate patient management.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Acute renal allograft rejections with major interstitial edema and plasma cell-rich infiltrates: High gamma-interferon expression and poor clinical outcome. Nephrol Dial Transplant. 2004;19:933-9.
- [Google Scholar]
- The clinical and pathologic implications of plasmacytic infiltrates in percutaneous renal allograft biopsies. Hum Pathol. 2001;32:205-15.
- [Google Scholar]
- Plasma cell-rich rejection processes in renal transplantation: Morphology and prognostic relevance. Transplantation. 2006;81:986-91.
- [Google Scholar]
- Posttransplant lymphoproliferative disorders: Summary of Society for Hematopathology Workshop. Semin Diagn Pathol. 1997;14:8-14.
- [Google Scholar]
- Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753-60.
- [Google Scholar]
- Acute interstitial nephritis of plasma cells: A new cause for renal allograft loss. Transplant Proc. 1993;25:897-9.
- [Google Scholar]
- Renal graft loss with plasma cell-rich acute rejection in cadaveric renal transplantation: A case report. Clin Transplant. 2005;19(Suppl 14):71-5.
- [Google Scholar]
- Immunoglobulin therapy for plasma cell-rich rejection in the renal allograft. Transplantation. 2006;82:567-9.
- [Google Scholar]
- Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754-61.
- [Google Scholar]
- C4d staining of renal allograft biopsies: A comparative analysis of different staining techniques. Nephrol Dial Transplant. 2007;22:568-76.
- [Google Scholar]
- Correlation between Banff classification, acute renal rejection scores and reversal of rejection. Kidney Int. 1996;49:481-7.
- [Google Scholar]
- Pathology and genetics of tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. :264.
- Kidney allograft with a lymphocytic infiltrate: Acute rejection, post transplantation lymphoproliferative disorder, neither or both entities. Am J Kidney Dis. 1997;30:449-54.
- [Google Scholar]
- Primer: Histopathology of polyoma virus-associated neohropathy in renal allografts. Nat Clin Pract Nephrol. 2006;2:531-6.
- [Google Scholar]







