Translate this page into:
Sevelamer carbonate experience in Indian end stage renal disease patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This open label, multicentric, comparative clinical trial was done to compare the efficacy and tolerability of two sevelamer formulations, sevelamer carbonate, and sevelamer hydrochloride, in the treatment of hyperphosphatemia in Indian end stage renal disease (ESRD) patients. A total of 97 ESRD patients on hemodialysis, were enrolled. Patients were randomized to receive either sevelamer carbonate or sevelamer hydrochloride. All patients were evaluated every week for 6 weeks for efficacy and safety variables. Total 88 patients completed the study. After 6 weeks of therapy, there were similar reductions (P<0.0001) in mean serum phosphorus and the CaxP product both the groups. The responder rates for test and reference groups were 75%, 68.18% respectively (P=0.3474). The adverse events reported were nausea, abdominal pain/discomfort, heartburn, constipation, diarrhea, increased prothrombin time, and severe arthritis. No serious adverse events were reported. There was no significant difference between the groups for adverse events and the laboratory parameters. From the results of this multicentric, comparative, randomized clinical study on sevelamer carbonate we can recommend that sevelamer carbonate may be used as a phosphate binder in Indian chronic kidney disease patients.
Keywords
Chronic renal failure
end stage renal disease
hyperphosphatemia
sevelamer carbonate
sevelamer hydrochloride
Introduction
Sevelamer hydrochloride is a nonabsorbed, calcium-free, aluminum-free phosphate binder for lowering serum phosphorus in patients with ESRD on hemodialysis.[1] Sevelamer is a cross-linked polymer that binds dietary phosphate ions within the gastrointestinal tract. It reduces phosphate absorption and thereby decreases serum phosphate concentrations without altering other electrolyte concentrations in patients with end stage renal disease (ESRD) who are on hemodialysis. Sevelamer is resistant to digestive degradation and is not absorbed across the gastrointestinal tract. Sevelamer also decreases the incidence of hypercalcemic episodes associated with calcium acetate treatment.[2–4]
Sevelamer carbonate is an improved, buffered form of sevelamer hydrochloride. Sevelamer carbonate has the same polymeric structure as sevelamer hydrochloride. The only difference in two molecules is the anion: carbonate versus chloride. This replacement provides alkali that may benefit chronic kidney disease (CKD) patients.[1]
The efficacy of the sevelamer carbonate in reducing serum phosphorus is expected to be the same as that of sevelamer hydrochloride, as the hydrochloride and carbonate salts have no role in phosphate binding. This study was conducted to compare the efficacy and tolerability of sevelamer carbonate hydrochloride in the treatment of hyperphosphatemia in Indian ESRD patients.
Patients and Methods
Study design
This was an open label, multicentric, and comparative clinical trial. A total of 97 patients were enrolled. All patients received either sevelamer carbonate (test) or sevelamer hydrochloride (reference). Patients were evaluated every week for 6 weeks for efficacy and safety variables. Study protocol was approved by the ethics committees of participating institutions. This study was performed in accordance with the principles laid down in Declaration of Helsinki.
Patient selection
Study population comprised of adult Indian ESRD patients undergoing hemodialysis, with a serum phosphorus level of ≥6 mg/dl. Patients were screened after obtaining the written informed consent for enrolment in the trial. Other phosphate binders, if any, were withdrawn for the period necessary before therapy. Patients with significant hypercalcemia or hypocalcemia (serum calcium >11.0 mg/dl or <7.9 mg/dl), significant concurrent illness, gastrointestinal disorders, malignancy, patients consuming medications containing aluminum, calcium, phosphorus, or magnesium, patients with clinically significant abnormal laboratory values (excluding markers of ESRD) and patients with known hypersensitivity to sevelamer were excluded. Also excluded were the women who were pregnant or lactating or of child bearing potential and not practicing effective methods of contraception.
Intervention
A patients were given a washout period of 2 weeks, during which no phosphate binder was administered. Randomization was carried out by using the software available online at www.randomization.com and using the words “test” for sevelamer carbonate and “reference” for sevelamer hydrochloride. Since, this was an open label study, no blinding was done. Then, either sevelamer hydrochloride (800 mg, three times a day) OR sevelamer carbonate (800 mg, three times a day or 1200 mg twice a day) treatment was initiated in all 97 patients as per the randomization. Dose of sevelamer was decided according to the serum phosphorus levels at the time of initiation of treatment. Duration of treatment was of 6 weeks.
The dosage regimen was as follows: For serum phosphorus >5.5 and <7.5 mg/dl, the dose was 2400 mg/day and for serum phosphorus ≥7.5 mg/dl, the dose was 4800 mg/day in divided doses. After every two-week intervals, the dose was to be increased (if serum phosphorus >5.5 mg/dl) or decreased (if serum phosphorus <3.5 mg/dl) by one tablet per meal.
Other phosphate binders were not allowed during the study. Patients were instructed to refrain from taking aluminum, calcium, or magnesium containing medications. However, vitamin D supplementation was allowed provided dose was kept constant throughout the study period. Any concomitant treatment taken by the patient was recorded in the case report form.
End points
The primary end point was the response rate. The responders were defined as those patients showing a reduction in serum phosphorus levels by at least 1 mg/dl, or having serum phosphorus value <5.5 mg/dl at the end of treatment.
The secondary end-points were reduction in serum phosphorus levels, reduction in serum calcium levels and reduction in the calcium × phosphorus product (calculated).
The safety end points were the occurrence of adverse events. During each visit, patients were monitored for changes in laboratory parameters of serum creatinine, urea, alkaline phosphatase, chloride, albumin, and bicarbonate.
Statistical methods
With the proposed sample size of 97 patients, the study would have power of 90% to detect a mean difference of 1.0 with a standard deviation of 1.5.[5] The sample size of 48 per group would also have 80% power to detect a difference of 25% between the groups.
The changes in serum phosphorus, serum calcium and Ca×P product were analyzed using Student's t-test. The responder rates were compared using Fischer's exact test. For all purposes, the “P” value less than 0.05 was considered as statistically significant.
A statistical analysis was performed using GraphPad InStat version 3.06 for Windows, GraphPad Software, San Diego California USA, Copyright 1992–1998 GraphPad Software Inc. (www.graphpad.com).
Results
Ninety-seven (68 male, 29 female) patients were enrolled over seven centers. The baseline parameters are provided in Table 1. Total 88 (63 male, 25 female) patients completed the study. The flow of the participants through the study is given in Figure 1.

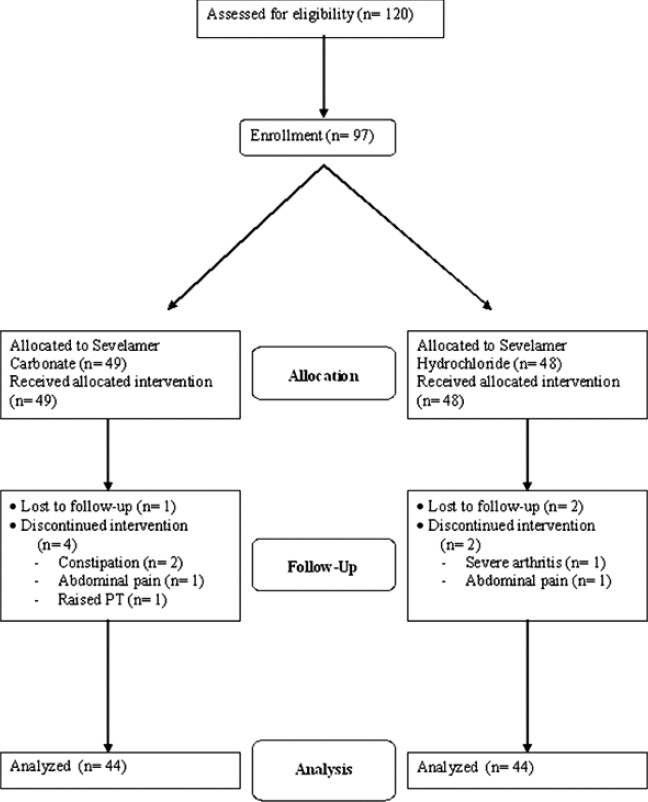
- Flow of the participants through study
Efficacy
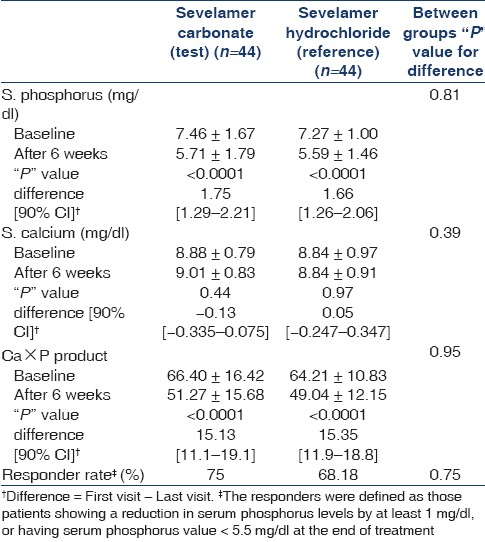
The changes in S. phosphorus, S. calcium, and Ca×P product and the responder rates (%) in both the groups are as given in Table 2.
Safety
There were no serious adverse events reported during the study period. The adverse events seen in sevelamer carbonate 800, sevelamer carbonate 1200, and sevelamer hydrochloride 800 groups were: nausea (6%, 0%, 2%, respectively), abdominal pain/discomfort (3%, 8%, 5%), heartburn (0%, 8%, 7%), constipation (6%, 8%, 0%), diarrhoea (0%, 8%, 0%), increased prothrombin time (3%, 0%, 0%), and severe arthritis (0%, 0%, 2%). After applying Fisher's exact test, no significant difference was found between the groups.
Discussion
Sevelamer carbonate is an anion exchange resin with the same polymeric structure as sevelamer hydrochloride in which carbonate replaces chloride. The replacement of the chloride with carbonate as the anion provides alkali that may benefit CKD patients.
A previous study[6] showed that sevelamer carbonate and sevelamer hydrochloride were well tolerated and equivalent in controlling serum phosphorus in patients with CKD on HD. A preclinical study[7] has shown that sevelamer may have important actions in decreasing diabetic and uremic vasculopathy and that sevelamer carbonate may be capable of increasing bone formation rates that are suppressed by diabetic nephropathy. Many studies have reported that sevelamer is associated with significantly less coronary artery calcification progression than calcium-based binders.[8–10] This ultimately results in lower mortality in dialysis patients.[11]
Sevelamer carbonate has been approved by US-FDA for the management of hyperphosphatemia in patients with chronic renal disease on hemodialysis. The purpose of this study was to assess the efficacy and safety of sevelamer carbonate in Indian CKD patients on hemodialysis who need phosphate binders.
The results of this Indian multicentric, comparative, randomized clinical study show that, in its ability to decrease mean serum phosphate levels in Indian CKD patients on hemodialysis, sevelamer carbonate is as effective (mean decrease=1.75 mg/dl, P<0.0001) as sevelamer hydrochloride (mean decrease=1.66 mg/dl, P<0.0001). In terms of responder rates sevelamer hydrochloride showed responder rate of 68.18%, while sevelamer carbonate showed responder rates of 70.97% (800 mg tablets), and 84.62% (1200 mg tablets) with mean of 75% (P=0.3474). It should be noted that frequency of hemodialysis affects this responder rate. In this study, the median frequency of hemodialysis was two sessions per week. Both the salts also decreased the Ca×P product value to below ideal goal level of 55 mg2/dl2.
The results of this study also show that the total daily dose of sevelamer carbonate, whether dosed in two or three divided doses, provides similar efficacy and tolerability. In fact, a study[1] suggests that even once a day dosing may be adequate.
The limitation of the study is the lack of PTH measurement and lack of data on vascular calcification. But the utility of such PTH values is difficult to judge given the wide interindividual, intraindividual variations and limitations of different assay technologies in quantifying iPTH levels.[12] Also the study was merely a noninferiority study and was not designed to compare the efficacy of the two formulations.
Both the salts (carbonate and hydrochloride) were equally well tolerated. The incidences of the side-effects are similar to that published for both the salts of sevelamer and as expected, majority of them are related to the gastrointestinal system.
Conclusion
From the results of this Indian multicentric, comparative, randomized clinical study on sevelamer carbonate we can recommend that sevelamer carbonate may be used as a phosphate binder in Indian chronic kidney disease patients.
The authors are thankful to Emcure Pharmaceuticals Ltd., Pune, India for providing the study medications.
This study was not registered as neither the registry was existent nor the registration was mandatory in India at the time of conduct of the study.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Novel dosage forms and regimens for sevelamer-based phosphate binders. J Ren Nutr. 2006;16:248-52.
- [Google Scholar]
- A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33:694-701.
- [Google Scholar]
- Long-term effects of sevelamer hydrochloride on the calcium×phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14:2907-14.
- [Google Scholar]
- RenaGel. A nonabsorbed calcium..and aluminum.free phosphate binder, lowers serumphosphorus and parathyroid hormone. The RenaGel Study Group. Kidney Int. 1999;55:299-307.
- [Google Scholar]
- Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42:96-107.
- [Google Scholar]
- A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol. 2007;68:386-91.
- [Google Scholar]
- Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122-30.
- [Google Scholar]
- Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant. 2005;20:1653-61.
- [Google Scholar]
- Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815-24.
- [Google Scholar]
- Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245-52.
- [Google Scholar]
- Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438-41.
- [Google Scholar]
- Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol. 2010;5:1261-7.
- [Google Scholar]







