Translate this page into:
Effect of reuse of polysulfone membrane on oxidative stress during hemodialysis
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with chronic renal failure, especially those on long-term hemodialysis (HD), have a high incidence of premature cardiovascular disease. Oxidative stress, which occurs when there is an excessive free radical production or low antioxidant level, has recently been implicated as a causative factor in atherogenesis. Hourly changes in malondialdehyde (MDA) and antioxidant enzymes, vitamins, lipid profile and ferric reducing ability of plasma (FRAP) were studied with the first use and immediate subsequent reuse of polysulfone dialysis membrane in 27 patients on regular HD treatment. Data were corrected for hemoconcentration and standardized to measure the rate of change. Increase in MDA and erythrocyte catalase along with decrease in plasma vitamin E and FRAP levels and no change in glutathione peroxidase levels were observed as a result of both fresh and reuse dialysis. These findings indicate a net oxidative stress in both fresh as well as dialyzer reuse sessions. There was no significant change in oxidative stress in both fresh and reuse sessions. The oxidative stress with reuse dialysis was less when compared to first use dialysis, but the difference was not statistically significant.
Keywords
Hemodialysis
oxidative stress
polysulfone membrane
reuse
Introduction
Chronic renal failure (CRF) is a state of increased oxidative stress (OS).[1] Oxidative stress is a state in which reactive oxygen species can cause oxidation of cellular and matrix macromolecules including sugars, proteins, deoxyribonucleic acid bases, and lipids. Increasing evidence suggests that OS may have a significant role in the development of complications in patients with end stage renal disease (ESRD) requiring dialysis. Patients with CRF, including those receiving regular long-term hemodialysis have high incidence of premature CVD. Oxidative stress plays an important role in the pathogenesis of vascular injury and progression of atherosclerosis and thus related to endothelial dysfunction (ED) and cardiovascular outcomes in patients with chronic kidney disease and patients on maintenance hemodialysis.[2]
Oxidative stress is assessed either by measuring markers of the oxidative damage to polyunsaturated fatty acids, such as malondialdehyde (lipid peroxides) or by inference from the levels of antioxidants. The antioxidants, which counter the attack of reactive oxygen species include intracellular antioxidant enzymes such as super oxide dismutase (SOD), glutathione peroxidase (GP), and catalase and non enzymatic chain-breaking antioxidants present in the plasma like vitamin E, A, and C. The majority of the studies have found increased lipid peroxidation in plasma and erythrocytes,[34] decreased plasma GP,[14] no change in plasma vitamin E,[5] and varying changes in erythrocyte SOD and GP.[67] These results point out to the likely presence of increased oxidative stress in patients on MHD.
Hemodialysis per se has also been shown to increase the oxidative stress, with reactive oxygen species being generated on the surface of the dialyzer membrane by the activation of polymorphonuclear leukocytes.[89] Even a single session of hemodialysis increases lipid peroxides and decreases antioxidants.[10–12] However, there are only a few reports on effect of reuse of the dialyzer membrane on oxidative stress.[1314] Most of the studies with the reuse of dialyzer are experimental studies probing into the reactive oxygen species production as an index of the polymorphonuclear leukocyte (PMNL) function[15] and the complement activation[16] as a result of reuse of the dialyzer rather than difference in the oxidative stress situation between fresh and reuse dialysis.
Hence, a time-course analysis of changes in MDA levels along with antioxidant enzymes and vitamins was taken up to evaluate oxidative stress as a result of hemodialysis with fresh use of the polysulfone membrane and during its subsequent reuse sessions.
Materials and Methods
Twenty-seven patients with end stage renal disease on maintenance hemodialysis for 3 months or more, were recruited after informed consent. Of these, 16 were males and 9 were females with a mean age of 50.4 ± 10.7 years (age range: 31–75 years). The causes of ESRD were diabetic nephropthy (40.7%), hypertensive nephropathy (29.6%), chronic glomerulonephritis (11.11%), ischemic nephropathy (3.73%), chronic interstitial nephritis (3.73%), and unknown (11.11%). Patients with acute infection were not included in the study group. The dialysis program consists of 4 h dialysis sessions three times a week.
Dialysis with the polysulfone membrane using bicarbonate dialysate was conducted with a dialysate flow rate of 500 ml/min and a blood flow rate of 200–250 ml/min. Water for the dialysate was purified by reverse osmosis. Anticoagulation was done with 2000 IU of heparin at the start of dialysis followed by continuous administration at a rate of 500–1000 IU/h. For each 4-h dialysis session, blood samples were collected at the start of dialysis (preHD), 1, 2, 3 h, and at the end of dialysis (post HD) from the arterial end of the dialyzer. Samples were centrifuged at 3000 rpm for 15 min, plasma separated and the erythrocytes were washed four times with saline before lysing with cold distilled water to prepare the hemolysate. Samples were analyzed either the same day or stored at –80°C until further analysis. Plasma samples were analyzed for malondialdehyde (MDA), triglycerides, vitamin E, ferric reducing ability of plasma (FRAP) while the hemolysate was used to measure erythrocyte glutathione peroxidase and catalase activities. Malondialdehyde, as an index of changes in lipid peroxidation, was estimated as thiobarbituric acid reactive substances (TBARS).[17] Vitamin E was measured by high-performance liquid chromatography.[18] Plasma total antioxidant capacity as ferric reducing ability of plasma,[19] glutathione peroxidase[20] (GP), and catalase[21] by continuous spectrophotometric rate determination. Triglycerides were estimated by standard methods using commercial kits on the Beckman coulter Synchron CX9 (Beckman coulter Inc., Fullerton CA) fully automated analyzer. Hemoglobin was estimated by the cyanmethemoglobin method using commercial kits.
Statistical analysis
Continuous variables were expressed in mean±SEM. values were corrected for hemoconcentration using creatinine, cholesterol, and albumin levels appropriate to the parameter. The data were transformed into percentage taking the predialytic value as 100% in order to nullify the effect of uremia and reflect change due to the dialysis session. Analysis of variance for repeated measures was performed to assess the overall time course change due to dialysis. A P value of <0.05 was considered significant. Statistical analysis was performed using Microsoft Excel and SPSS version 11.5 for Windows.
Results
Baseline characteristics of patients is given in Table 1. The mean±SE of the oxidant and antioxidant parameters is given in Table 2. The direction of change along with significance of the parameters studied is given in Table 3. A significant increase in plasma MDA was found with both fresh and reuse (P<0.05), along with significant decrease in the antioxidants like Vitamin E and FRAP (P<0.05). The catalase activity showed a significant increase while no change in the GPX activity was observed in fresh or reuse sessions.



Figures 1–3 show the time course changes of these parameters as a result of dialysis with fresh use and subsequent reuse. As shown in Figures 1a and b, an increase in plasma MDA levels is seen with both fresh use and subsequent reuse with the polysulfone membrane and is reflected in the changes is triglycerides levels that showed a decrease as a result of both fresh and subsequent immediate reuse. The antioxidants especially plasma Vitamin E and FRAP levels showed a decrease as a result of both fresh and subsequent immediate reuse sessions [Figure 2a and b], while an increase in the catalase activity was seen with in fresh and the reuse session. However, no significant change in the GPX activity was observed as a result of both fresh and reuse of the polysulfone membrane [Figure 3a and b].
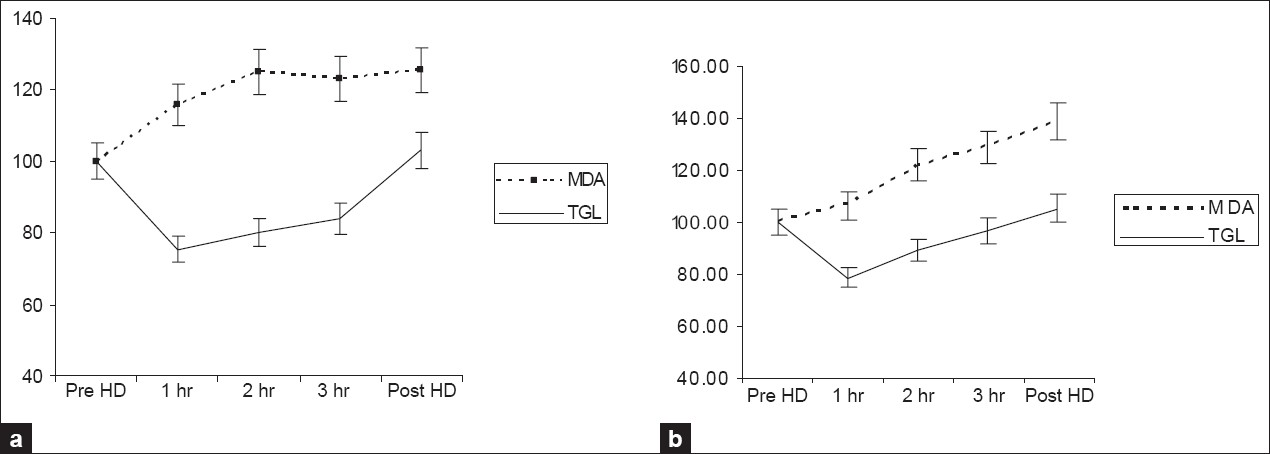
- Time course of changes in the parameters study during a 4 h dialysis with the polysulfone membrane. Data collected hourly during dialysis were corrected for hemoconcentration and converted to percentages with the predialytic value taken as 100%. The results are presented as mean±SEM (n=27). 1a and 1b. Changes in MDA and TGL with fresh and reuse, respectively; MDA: Malondialdehyde, TGL: Triglycerides

- Time course of changes in the parameters study during a 4 h dialysis with the polysulfone membrane. Data collected hourly during dialysis were corrected for hemoconcentration and converted to percentages with the predialytic value taken as 100%. The results are presented as mean±SEM (n=27). 2a and 2b changes in vitamin E and FRAP with fresh and reuse, respectively FRAP-ferric reducing ability of plasma

- Time course of changes in the parameters study during a 4 h dialysis with the polysulfone membrane. Data collected hourly during dialysis were corrected for hemoconcentration and converted to percentages with predialytic value taken as 100%. The results are presented as mean±SEM (n=27). 3a and 3b. Changes in GPX and CAT with fresh and reuse, respectively. GPX-glutathione peroxidase, CAT-catalase
Discussion
Plasma malondialdehyde (MDA) is a small water-soluble molecule and is cleared by the dialysis.[11] Hence, if there is no generation, the levels of MDA are expected to decrease like creatinine at the end of the dialysis session. CKD and ESRD represent a state of increased OS attributed to the uremic state, chronic infections, malnutrition, and iron overload. These factors are likely to act as confounding factors. In order to overcome this, we have converted the data into percentage taking predialytic levels as 100% so as to measure only the changes that occur due to dialysis. A significant increase in MDA levels were observed after correcting for creatinine (P<0.05), suggesting production of malondialdehyde during dialysis, pointing toward generation of free radicals. It supports the view that plasma MDA is both generated and cleared during dialysis.[101122]
Most of the studies that reported an increased oxidant stress found that the effect was observed mainly during the first hour of dialysis,[13] and in accordance we also observed that the rise in plasma MDA levels corrected for clearance was more in the first hour of dialysis when compared to the later phase of dialysis.[132324] Reuse of membranes is said to be associated with less complement activation than fresh membranes as a result of fixation of plasma proteins to the membranes and hence lead to less ROS production.[12] We found an increase in MDA as a result of reuse of the membrane (P<0.05).
The main lipid substrates for oxygen-free radicals are unsaturated fatty acids in the triglycerides. The increase in MDA in the first hour is paralleled by a decrease in triglycerides [Figure 1a], which is the evidence for production of MDA from triglycerides. In our study, the time course of changes in plasma triglycerides levels showed a decrease as a result of both fresh (P<0.001) and subsequent immediate reuse (P<0.001) with the polysulfone membrane. This is in agreement with the literature.[25]
Formation of MDA is a chain event as the MDA formed attack more fatty acids. This chain of events is broken by chain breaking antioxidants, vitamin E, thiols, and vitamin C. Vitamin E prevents lipid per oxidation by being preferentially oxidized instead of fatty acids and gets consumed in this process. Therefore, a decrease in the levels is due to consumption in its attempt to reduce the effect of oxygen free radicals generated during hemodialysis. In our study, the time course of changes in plasma Vitamin E levels showed a decrease as a result of both fresh (P<0.001) and subsequent immediate reuse (P<0.012) dialysis with the polysulfone membrane.[10]
Total antioxidant capacity is an indicator of plasma resistance against oxidant agents. The major contributors to the total antioxidant capacity of plasma are urate, ascorbate, vitamin E, and plasma protein.[26] We have measured this status in the form of ferric reducing ability of plasma (FRAP). A significant decrease in fresh (P=0.001) and reuse (P<0.001) in FRAP levels were observed in our study, which is in accordance with earlier reports.[2728]
Super oxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT), together with glutathione (GSH), form the main line of defense against ROS in erythrocytes. Super oxide dismutase dismutases the super oxide radicals to hydrogen peroxide. Increase in formation of H2O2 as a result of dismutation of superoxide radicals by SOD causes the increase activity of GPX and catalase. However, due to the difference in Km, the contribution of GPX and CAT in detoxification of H2O2 is different. GPX acts at low H2O2 concentration whereas CAT plays a role when GPX pathway reaches saturation. A significant increase in catalase activity was seen with fresh (P=0.001) and reuse (P<0.001) without any change in the GPX activity in either fresh (P=0.704) or with reuse (P=0.301) of the dialyzer. However, low catalase activity was reported by Mimc–Oka et al.[1] The increase in the catalase activity observed in our study might be due to low levels of GPX observed in dialysis patients coupled with increased generation of hydrogen peroxides above the capacity of GPX. Data regarding the GPX activity in HD are conflicting. Some studies have reported an increase in the GPX activity while others have reported no change.
Thus, our study shows increase in oxidative stress with both fresh and reuse dialysis. Moreover, there was no significant decrease in oxidative stress with reuse of the dialyzer membrane. As shown in Figure 1a, maximum increase in MDA levels occurred during the first hour of dialysis. However, with reuse [Figure 1b], increase occurred from second hour onwards, which indicates the possible role of facors other than between blood and membrane interaction. These finding are supported by the change in vitamin E levels, the immediate chain breaking antioxidant. A sharp decrease in vitamin E levels occurred at first hour coinciding with the increase in MDA levels with fresh use of the dialyzer [Figure 2a], but the decrease seen with reuse was uniform, though it shows an increase at 1 h [Figure 2b], that was found to be statistically insignificant (data not shown). Observation of data shown in Table 2 shows a lesser degree of oxidative stress with reuse of the dialyzer when compared to fresh use of the dialyzer. This may be due to a slightly better antioxidant status as observed by the levels of Vit E, FRAP, and catalase in reuse of the dialyzer when compared to their status in fresh use of the dialyzer [Figures 3a and b] and is able to better combat the load of free radicals generated during the reuse session. This is in agreement with previous reports that demonstrated a lesser degree of oxidative stress with reuse of the dialyzer.[29] Dialyzer reuse has been shown to have beneficial effects on whole blood superoxide anion generation[13] and cause an improvement in oxidative stress.[14]
Conclusions
The results of our study with fresh use dialyzer show a net oxidative imbalance in the form of increase in plasma MDA levels, catalase activity, and a decrease in plasma vitamin E and FRAP levels as a result of dialysis session. Hence, it may be concluded that a single session of hemodialysis adds to the OS of the uremic state. However, despite evidence in the literature for persistence of oxidative stress with reuse no increase in mortality risk was observed in patients.[30–33]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Alterations in plasma antioxidant capacities in various degrees of chronic renal failure. Clin Nephrol. 1999;51:233-41.
- [Google Scholar]
- Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153-7.
- [Google Scholar]
- Increased lipid peroxidation in patients on maintenance haemodialysis. Nephron. 1992;60:56-9.
- [Google Scholar]
- Evaluation of oxidative stress in haemodialysis patients: Use of different parameters. Clin Chem Acta. 1995;234:109-14.
- [Google Scholar]
- Determination of erythrocyte superoxide dismutase, glucose-6-phosphate dehydrogenase, reduced glutathione and malondialdehyde in uremia. Clin Chim Acta. 1982;123:153-9.
- [Google Scholar]
- Antioxidant enzymes-super oxide dismutase and gluatatione peroxides-in hemodialyzed patients. Blood Purif. 1996;14:257-61.
- [Google Scholar]
- Dysfunction of polymorphonuclear leukocytes in uremia. Semin Nephrol. 1996;16:192-201.
- [Google Scholar]
- Effect of haemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin Nephrol. 1997;44:1135-8.
- [Google Scholar]
- Influence of dialysis on plasma lipid peroxidation products and antioxidant levels. Kidney Int. 1996;50:1268-72.
- [Google Scholar]
- Oxidative stress in hemodialysis: Immediate changes caused by passage of blood through the dialyser. Ann Clin Biochem. 2001;38:401-5.
- [Google Scholar]
- Superoxide anion generation and lipid peroxidation processes during hemodialysis with reused cuprophan dialyzers. Free Radic Biol Med. 1990;8:429-32.
- [Google Scholar]
- Oxidative stress in hemodialyzed patients and the long-term effects of dialyzer reuse practice. Clin Biochem. 1997;30:601-6.
- [Google Scholar]
- Dialyzer membrane type and reuse practice influence polymorphonuclear leukocyte function in hemodialysis patients. Kidney Int. 2004;65:682-91.
- [Google Scholar]
- Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol. 2002;13:62-71.
- [Google Scholar]
- Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radical Biol Med. 1990;8:15-9.
- [Google Scholar]
- Simultaneous determination of retinol and a tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983;29:708-12.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal l Biochem. 1996;239:70-6.
- [Google Scholar]
- GPX. In: Jakoby W.B, ed. Methods in ezymology-77 NY. Waltham, Massachusetts: Academic Press; 1981. p. :325-33.
- [Google Scholar]
- Lipoperoxidation in plasma andred blood cells of patients undergoing hemodialysis: Vitamins A, E, and iron status. Free Radic Biol Med. 1994;16:339-46.
- [Google Scholar]
- Enhancement of reactive oxygen species production and cell surface markers expression due to haemodialysis. Nephrol Dial Transplant. 1994;9:389-94.
- [Google Scholar]
- Cuprophane haemodialysis induces upregulation of LPS receptor (CD14) on monocytes: Role of complement activation. Nephrol Dial Transplant. 1996;11:657-62.
- [Google Scholar]
- The role of heparin in the changes of lipid pattern during a single hemodialysis. Clin Neprol. 1982;17:96-9.
- [Google Scholar]
- The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987;924:408-19.
- [Google Scholar]
- Assessment of plasma antioxidant status in hemodialysis patients. Ther Apher Dial. 2008;12:147-51.
- [Google Scholar]
- Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: A possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res. 2000;47:618-23.
- [Google Scholar]
- The effect of hemodialysis with frequent use of cuprophan and polysulfone membranes on activation of complement in patients with chronic renal failure. Pol Arch Med Wewn. 1996;96:458-68.
- [Google Scholar]
- Reuse-associated mortality in incident hemodialysis patients in the United States, 2000 to 2001. Am J Kidney Dis. 2005;46:661-8.
- [Google Scholar]
- Increased free-radical activity during hemodialysis. Neprol Dial Transplant. 1987;2:169-71.
- [Google Scholar]
- Abnormal antioxidant vitamin and carotenoid status in chronic renal failure. Q J Med. 1996;89:765-9.
- [Google Scholar]







