Translate this page into:
Nondiabetic kidney disease in type 2 diabetic patients: A single center experience
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nondiabetic renal disease (NDRD) is seen as a cause of proteinuria and renal failure in type 2 diabetes mellitus (DM). The clinical differences between NDRD and diabetic glomerulosclerosis (DGS) are not clear. This study was done to find the spectrum of NDRD in type 2 DM patients and differences in clinical profile between NDRD and DGS patients. Data of patients with type 2 DM who underwent renal biopsy in this institute from 1990 to 2008 were analyzed retrospectively. Patients were categorized as isolated NDRD, NDRD with DGS, and isolated DGS. A total of 75 patients were included. Mean age was 45 ± 10.2 years, male to female ratio was 3.1 : 1, median duration of DM was 12 months (range, 1 year-15 years), proteinuria was 4.2 ± 3.4 g/day, and serum creatinine was 4.3 ± 3.9 mg/dl. Hypertension was observed in 63 (84%) cases and microscopic hematuria in 24 (32%) cases. Nephrotic syndrome (38.7%) was the commonest clinical presentation. Forty-eight (64%) cases had NDRD and 27 (36%) had DGS. The commonest NDRD was minimal change disease (12.5%). Three (6.3%) patients had lupus nephritis. Tubulointerstitial nephritis has been observed in 10.4% patients. No significant differences between NDRD and DGS patients were found except hypertension which was significantly high in the DGS group. Acute kidney injury and nephritic syndrome were not observed in the DGS group. In conclusion, the incidence of biopsy-proven NDRD in type 2 DM in this study was high. Kidney biopsy aided in the detection of NDRD in clinically suspected patients.
Keywords
Kidney biopsy
nondiabetic renal disease
type 2 diabetes
Introduction
Proteinuria in diabetic patients is usually interpreted as a clinical manifestation of diabetic nephropathy (DN). However, not all diabetic subjects with proteinuria have DN. Nondiabetic renal disease (NDRD) has been seen to cause proteinuria in diabetic patients. There is a wide variation of prevalence of NDRD. The occurrence of NDRD in type 1 diabetes mellitus (DM) is rare in comparison with those with type 2DM. Although exact incidence of NDRD is not known, frequency varies from 5% to 71% in various studies.[1] It is seen in 26.7% of Asian and 22% of European patients.[2–4] Kidney biopsy is an unbiased method, but is seldom used in proteinuric diabetic patients. Olsen, in a meta-analysis of similar studies, found the frequency of glomerulonephritis (GN) to be between 0 and 66%. These variation is probably due to variable selection criteria and geographical differences.[5] Late age of onset of DM, absence of neuropathy, absence of retinopathy, and presence of other systemic diseases are reported as markers of NDRD in different studies. However, it remains unclear which clinical factors have greater value in the prediction of NDRD. As the reported incidence of NDRD in type 2 DM is high, it is necessary to predict, diagnose, and treat the concurrent glomerular diseases because of the prognostic and therapeutic importance.[6] So far, only few such studies had been published from India. We carried out this study to find the clinical, laboratory, and pathological features of NDRD in type 2 DM patients and also to see any significant differences in clinical profile between the NDRD and DGS groups.
Materials and Methods
The demographic, clinical, and biochemical data of patients with type 2 DM (defined by the American Diabetes Association) who underwent renal biopsy in this institute from 1990 to 2008 were analyzed retrospectively.[7] Data were collected from histopathological reports, requisition forms, and discharge summaries. Post-transplant patients and patients with lack of adequate clinical data and inadequate renal biopsies were excluded. The indications for renal biopsy in diabetic patients with clinically suspected NDRD included persistent hematuria, active urinary sediment, rapidly progressive renal failure (RPRF), sudden onset of nephrotic syndrome (NS), asymptomatic urinary abnormalities in the absence of diabetic retinopathy (DR), and other microvascular disease, renal insufficiency of unexplained origin, etc. If patients had a long history of diabetes with severe signs of multi organs involvement such as retinopathy and other microvascular diseases, the diagnosis of DN was considered obvious and renal biopsy deferred. Renal biopsy was performed by using tru-cut needle prior the year 2000 and whereas automated biopty gun were used from the year 2001 onward. Tissue was processed for light microscopy (using Hematoxylin and eosin, periodic acid Schiff, Masson's trichrome, and Jones silver methenamine staining), immunofluorescence (by using FITC-conjugated rabbit antihuman antibody sera, DAKO, Denmark against human IgG, IgM, IgA, C3, C1q, kappa and lambda light chains). The biopsies were reported by one and reviewed by two pathologists of the institute. Both used same reporting criteria. Electron microscopy was not used as this facility is not available in our institute.
Diabetic glomerulosclerosis (DGS) was diagnosed by the presence of mesangial expansion, with or without the nodular Kimmelstiel – Wilson (KW) formation, basement membrane thickening, fibrin caps, or capsular drops. Vascular changes of DN included arteriolar hyalinosis, medial hyperplasia of smaller arteries, and intimal sclerosis of larger arteries. NDRDs were categorized as per WHO classification of 1995.[8]
Based on the biopsy findings, patients were categorized as isolated NDRD, NDRD with underlying DGS, and isolated DGS groups.
Following definitions were used:[910]
Nephrotic syndrome: NS was defined as proteinuria >3.5 g/day/1.73 m2 body surface area with edema and/or hypoalbuminemia (serum albumin <3.5 g/dl), without Acute kidney injury (AKI), with or without hematuria.
Asymptomatic urinary abnormalities: Proteinuria without NS was defined as <3.5 g/day. All patients in this group were without significant decline in renal function.
Hematuria: Microscopic hematuria was defined as >5 erythrocytes per high-power field on microscopic examination of the urine without significant decline in renal function.
Acute kidney injury: Abrupt onset of failure of renal function leading to retention of nitrogenous waste products; often anuric or oliguric.
Chronic renal failure (CRF): End result of variety of progressive /irreversible renal disease; accompanied by uremia when elevated serum creatinine (>1.5 mg/dl) persisted for >6 months.
Acute Nephritic syndrome: defined as hematuria, red cell cast, hypertension, oliguria, edema, proteinuria <3 g/day, and reduced glomerular filtration rate, abrupt onset.
Rapidly progressive renal failure: defined as renal failure over days/weeks, proteinuria usually <3 g/day, hematuria, red cell cast, blood pressure often normal, may have other features of vasculitis.
Hypertension was considered when blood pressure was higher than 130/90 mm Hg.
Statistical analysis
Descriptive statistical methods were used. For metric data, arithmetic mean, standard deviation, and median were used. For categorical data, Fisher's exact test was applied on 2 by 2 analyses in contingency table using statistical web page www.statpages.org. Student T-test was used for continuous variables. A P value <0.05 was considered as statistically significant.
Results
A total 1,849 patients underwent renal biopsy during this period. The number of biopsies done on type 2 diabetic patients for suspicion of NDRD was 105, of which 30 were excluded (23 cases had renal allograft biopsy and 7 had no adequate data). A total of, 75 patients were included. The mean age was 45 ± 10.2 years, male to female ratio was 3.1 : 1, median duration of DM was 12 months (range, 1 year - 15 years), mean proteinuria was 4.2 ± 3.4 g/day (median, 3.1 g/day [range, 0.35 to 12 g/day]) and mean serum creatinine was 4.3 ± 3.9 mg/dl (range, 0.8 to 22 mg/dl [median, 3.1 mg/dl]). Hypertension was observed in 63 (84%) cases. Urinalysis revealed Microscopic hematuria was noted in 24 (32%) cases. The renal syndromes at presentation are shown in Figure 1. The commonest one was NS. Though clinically suspected as NDRD, 27 (36%) cases were diagnosed to have DGS, of which 12 had KW nodules. The distribution of histological patterns of NDRD (n = 48) patients were shown in Table 1. The commonest primary glomerular disease (PGD) was minimal change disease (MCD) (12.5%). The incidence of both secondary glomerular disease and tubular interstitial nephritis (TIN) was 10.4% [Table 1].

- Clinical Syndrome. AKI; acute kidney injury, ANS; acute nephritic syndrome, AUA; abnormal urinary analysis, CRF; chronic renal failure, NS; nephrotic syndrome, RPRF; rapidly progressive renal failure

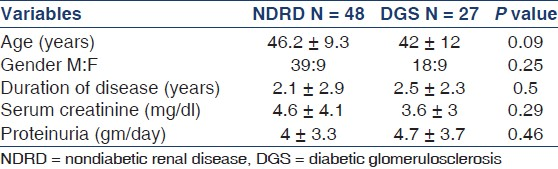
The frequency of NDRD of total renal biopsies on diabetic patients was 64% (n = 48). Three patients had NDRD on superimposed DGS as post-infectious GN (PIGN), IgA nephropathy (IgAN), and chronic interstitial nephritis, one in each category and they were clubbed with NDRD group. In comparison with the clinical and laboratory data, no significant differences between NDRD and DGS patients were found except hypertension that was significantly high in the DGS group [Table 2]. Raised serum creatinine and microhematuria were more frequent in NDRD group but statistically not significant. The commonest clinical presentation in both groups was NS followed by RPRF [Table 3]. The results of NS and RPRF were further analyzed separately [Table 4]. DGS was the most common histological finding in the NS group, whereas crescentic GN was commonest finding in the RPRF group [Table 4].


Discussion
In this study of a selected population, the incidence of NDRD was 64%. This result is similar to that reported in India and other regions where incidence was more than 50%.[11–17] On the other hand, some other studies from India and also other parts of the world showed a very low incidence.[218–29] This discrepancy of variable prevalence is most likely due to different biopsy policies, geographic and ethnic factors. Similarly, the incidence of NDRD superimposed on DGS was also variables as reported by Soni et al. (30%), Chien-Pin Lai (40%), Hashim Ali J and Saedi (19.3%), Pham et al. (40%), Muzzucco et al. (17%), and Atsuhito Tone et al. (16.5%) in their respective studies, which is quite different from our study (4%).[121416172027]
NS, RPRF, and AKI were the most frequent clinical presentation in our study. This is agreeing with majority of the published studies.[11–1329] Contrary to this, Prakash et al. reported CRF as the most common clinical presentation (47%) followed by ANS (18.7%) and NS (15.6%).[2] A recent study from Taiwan observed AKI(58.8%) in majority of patients while in Kuwait, it was hematuria.[1426] From these review of literatures, it is obvious that presenting syndrome can be different.
Many reported studies deny any clinical and laboratory correlation that predict NDRD in diabetic population, particularly in relation with proteinuria, absence of retinopathy, neuropathy, age of onset, duration of DM, and renal function.[13142129–31] Unlike these, Mak et al. and Lee et al. observed microscopic hematuria as an important predictor of NDRD which is also supported by a recent study from China.[132132] The frequency of hematuria in the present study is more in the NDRD (36% vs 23%) group but not statistically significant. Controversy exists with regards to absence of DR as an indicator for NDRD. According to Pham et al., absence of DR is a predictor of NDRD.[17] However, others considered it as a poor indicator since the chance of DGS and NDRD was 50%.[33] A previous study from India too reported that 50% cases of clinically suspected NDRD that turned out to be DGS.[18] Similar results reported by few other studies where in fact incidence of DGS was more than 60%.[212931] In contrast, DGS was noted only in 36% cases in the present study, where all patients were negative for DR. Several studies from India suggest that in suspected cases, absence of DR is a good indicator for NDRD.[1112] However, it must be remembered that even among patients suspected to have NDRD, approximately 50% could have diabetic glomerulopathy.
In the present study, while comparing data of NDRD patients with DGS patients, no significant difference in baseline, clinical, and biochemical data was observed except for hypertension, which is more in the DGS group. It is worth to mention here that important data regarding HbA1c, lipid profile, were not found due to retrospective nature of the study. We observe that AKI and ANS rarely present in DGS group. Due to smaller number of sample size, we did not get a statistical significant value. Unlike described by Soni et al., we have not observed significant difference in mean duration of disease between the two groups.[12] This can be explained by the more selective and biased nature of our study. A recent Chinese study has however identified duration of DM, systolic blood pressure, HbA1c, hematuria, and retinopathy as the five major differential indicators.[32]
PGD was the most common inferred renal pathology among all types of NDRD with type 2 DM. Almost all types of GN have been reported.[36] In the present study, MCD is the commonest PGD followed by PIGN, chronic GN, membranous nephropathy (MN), and Crescentic GN (CresGN) which is not in line with other reported series from India. Soni et al. showed acute interstitial nephritis as the most common NDRD.[12] Prakash et al. from north India also reported a high incidence of TIN (53.2%) and another study from south India observed proliferative GN as the most common change.[211] However, Premlata et al. found MN as the most common pathologic change.[18] In contrary to these, Hashim et al. from Iraq reported MPGN (40%) as the commonest followed by focal segmental glomerulosclerosis (FSGS) (20%), MN (25%), MCD (10%), and amyloidosis (5%).[16] In a study from Italy where Mazzucco et al. analyzed kidney biopsies of 393 patients of type 2 DM observed MN (28.4%) as the most common PGD followed by IgAN (22%), MCD/FSGS (20%), and PIGN (10.1%).[20] Quite contradicting to the present series, studies from Korea and China reported IgAN as the commonest NDRD accounted for 59% patients.[1321] A recent Chinese study also reported similar result.[32] It is to be noted that IgAN is the most common PGD in these countries. Incidence of IgAN of the total PGD in our study is only 6%.[34] Hence, its frequency in NDRD group is also low. A study from Thailand showed MN as commonest NDRD, while in Kuwait, it was CresGN.[2629] Conversely, Shan and Pham reported FSGS as the most common NDRD.[1517] These results suggest that prevalence of different category of biopsy-proven renal disease in diabetic patients depends on the usual prevalence of renal disease in the total population according to the geographical area and ethnic characteristics and NDRD is merely a coincidental in type 2 DM.
To conclude on the basis of this study NDRD is frequent in type 2 DM. We believe that it is difficult to differentiate NDRD from DGS merely on the basis of clinical and laboratory criteria, though absence of DR, microhematuria, AKI, nephritic presentation could be considered as indicators of NDRD. Hence, kidney biopsy is an important diagnostic tool to define underlying renal disease other than DGS in type 2 DM patients with proteinuria and/or sudden decreasing of renal function with absence of DR, which also has prognostic and therapeutic importance.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Diabetic Nephropathy.Heptinstall's Pathology of the Kidney. (6th ed). Philadelphia: Lippincott Williams & Wilkins; 2007. p. :801-52.
- [Google Scholar]
- Non-diabetic renal disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2001;49:415-20.
- [Google Scholar]
- Renal outcome in type 2 diabetic patients with or without coexisting non-diabetic nephropathies. Diabetes Care. 2002;25:900-5.
- [Google Scholar]
- How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638-45.
- [Google Scholar]
- Identification of non-diabetic glomerular disease in renal biopsies from diabetics—a dilemma. Nephrol Dial Transplant. 1999;14:1846-9.
- [Google Scholar]
- Renal biopsy as a guide to the treatment of glomerulonephritis. The therapy in nephrology and hypertension. In: Brady HR, Willox CS, eds. A companion to Brenner and Rector's. The Kidney (5th ed). Philadelphia Pa: WB Saunders; 1999. p. :85.
- [Google Scholar]
- American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62-7.
- [Google Scholar]
- Renal Disease: Classification and atlas of glomerular disease. (2nd ed). New York: Ikagu Shoin medical publishers Inc; 1995. p. :1-359.
- [Google Scholar]
- Introduction to glomerular disease: Clinical presentations.Comprehensive clinical nephrology. (3rd ed). Philadelphia: Mosby Elsevier; 2007. p. :193-207.
- [Google Scholar]
- The Kidney. The pathologic Basis of Disease. (5th ed). Philadelphia: WB Saunders; 1994. p. :932.
- [Google Scholar]
- Nondiabetic renal disease in Noninsulin- dependent diabetics in a South Indian Hospital. Nephron. 1994;67:441-3.
- [Google Scholar]
- Nondiabetic Renal disease in Noninsulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321-6.
- [Google Scholar]
- Non-diabetic renal diseases in type 2 diabetic patients with renal involvement: Clinicopathological study in a single medical center in Taiwan. Acta Nephrologica. 2010;24:157-66.
- [Google Scholar]
- Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;87:3354-9.
- [Google Scholar]
- Pathology of nondiabetic glomerular disease among adult Iraqi Patients from a single centre. Saudi J Kidney Dis Transpl. 2009;20:858-61.
- [Google Scholar]
- Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322-8.
- [Google Scholar]
- Prevalence of non-diabetic renal disease in type 2 diabetic patients in a diabetes centre in Southern India. J Assoc Physicians India. 2002;50:1135-9.
- [Google Scholar]
- An evaluation of renal biopsy in type-II diabetic patients. J Coll Physicians Surg Pak. 2009;19:627-31.
- [Google Scholar]
- Different patterns of renal damage in type 2 diabetes mellitus: A multicentric study on 393 biopsies. Am J Kidney dis. 2002;39:713-20.
- [Google Scholar]
- Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588-91.
- [Google Scholar]
- Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant. 1992;7:397-9.
- [Google Scholar]
- Increased prevalence of nondiabetic renal pathology in type 2 diabetes mellitus. Nephrol Dial Transplant. 1992;7:1258-9.
- [Google Scholar]
- Clinical characteristics of non-diabetic renal disease in Type 2 diabetic patients. Korean J Nephrol. 2004;23:949-56.
- [Google Scholar]
- The morphologic patterns of diabetic nephropathy in Koreans. J Pathol. 2009;43:36-42.
- [Google Scholar]
- Renal biopsy in patients with type 2 Diabetes Mellitus: Indication and Nature of Lesions. Bull Alex Fac Med. 2009;45:343-7.
- [Google Scholar]
- Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69:237-42.
- [Google Scholar]
- Nondiabetic Renal Disease in Type 2 Diabetic Patients: A Review of our Experience in 220 Cases. Ren Fail. 2011;33:26-30.
- [Google Scholar]
- Non-diabetic glomerular disease in Type II DM: 10 years experience. J Med Assoc Thai. 2009;92:S57-60.
- [Google Scholar]
- Clinical identification of non-diabetic renal disease in diabetic patients with type I and type II disease presenting with renal dysfunction. Am J Nephrol. 1988;8:204-11.
- [Google Scholar]
- Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719-31.
- [Google Scholar]
- A differential diagnostic model of diabetic nephropathy and non-diabetic renal disease. Nephrol Dial Transplant. 2008;23:1940-5.
- [Google Scholar]
- Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758-62.
- [Google Scholar]
- Pattern of biopsy proven renal disease in a single center of South India: 19 years experience. Indian J Nephrol. 2011;21:250-7.
- [Google Scholar]







