Translate this page into:
Nephrotic syndrome associated with meningioma
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 58-year-old man presented with recurrent frontal meningioma and nephrotic syndrome. Renal biopsy could not be done in view of the rapid neurological deterioration. The patient underwent surgical resection of the tumor. Within 4 weeks, the edema decreased, serum albumin improved, and proteinuria decreased spontaneously. At three months of followup, the patient had attained complete remission of nephrotic state.
Keywords
Meningioma
Paraneoplastic nephrotic syndrome
resolution of nephrotic syndrome after tumor resection
Introduction
A variety of renal diseases are associated with tumor or its treatment with chemotherapeutic drugs or radiation.[1] The pathogenesis of nephrotic syndrome in patients with malignancy is not understood. Tumors, being an important source of antigens, may induce the production of specific antibodies which form immune complexes, which subsequently deposit in the kidneys. Another postulation is that, antigens with a high affinity for basement membrane constituents can get implanted directly into the renal tissue and induce the formation of immune complexes with circulating antibodies.[2] These two mechanisms would explain the role of chemotherapy or surgery in the reversal of the glomerular injury in these settings. In a recent study, tumor-bearing rats developed features of glomerulopathy, and this animal model may provide new insights into the development of paraneoplastic glomerulopathies.[3]
Nephrotic syndrome has been associated with solid tumors and hematologic malignancies. The association of glomerulopathy and a malignancy can be supported by at least two facts. First, a remission of proteinuria may be achieved after surgical removal of tumor or disappearance of the tumor with chemotherapy. Second, relapse of proteinuria may occur after recurrence of the malignancy. The most common renal association in a patient with solid tumor and nephrotic syndrome is membranous nephropathy. There is a significant increase in incidence of cancer among patients with membranous nephropathy when compared to the general population.[14]
Nephrotic syndrome, although described in association with various malignancies, is not known to be associated with neurological tumors. We present a patient with a benign intracranial tumor (meningioma) who presented with nephrotic syndrome, which went into remission after surgical removal of the tumor. To the best of our knowledge, nephrotic syndrome has not been described so far in association with meningioma.
Case Report
A 58-year-old man presented to the nephrology clinic in September 2011 with a history of progressive pedal and scrotal edema of two weeks’ duration. In 2005, he had undergone surgical resection of a left frontal meningioma and follow-up till 2007 was unremarkable. Since the last 12 months, he had gradually increasing proptosis on the left side with decreasing vision, which had significantly worsened since the last two weeks. There was no history of any drug intake during the last 12 months.
Physical examination revealed massive pedal and scrotal edema, left orbital proptosis with mechanical restriction of left-eye movement, mainly elevation and abduction, and chemosed conjunctiva. There were no other neurological deficits; other systems were normal. Direct fundoscopy revealed a large mass indenting almost half the globe from above with multiple choroidal folds Passing through the fovea with dilation and tortuosity of retinal veins, predominantly in the superior half with disc edema on the left side.
He had normal serum creatinine (0.6 mg/dl), hypoalbuminemia (1.5 gm/dl), hypercholesterolemia (300 mg/dl) and proteinuria (4+) with no abnormality on urine microscopy. 24-h urine protein excretion was 12.672 mg. Hemogram, blood sugars, and serum complements were normal. HBsAg and anti-HCV (ELISA) was negative. Ultrasonogram of the abdomen showed normal-sized kidneys with bilateral, raised cortical echo texture. X-ray chest was normal. A diagnosis of nephrotic syndrome, probably membranous nephropathy was entertained. Computed tomography (CT) scan of head showed a large-mass lesion involving the frontal and intra-orbital regions with extensive bony involvement [Figure 1]. As per the imaging characteristics, the lesion was very suggestive of frontal meningioma.

- Computed tomography scan of head showing a heterogeneously enhancing 5 × 4.2 × 1.9 cm sized extradural lesion (arrow) along the left frontal bone and roof of the orbit on left side extending anteriorly
The patient was initially treated with diuretics and albumin/plasma transfusions. A renal biopsy was planned; however, he developed recurrent focal seizures. He was started on appropriate anti-convulsants. Despite being on anti-convulsants, he had an episode of status epilepticus, consequent to which he had to be ventilated. In view of the recurrent seizures, he had to be taken up for an emergency surgery. He underwent left craniotomy, and total excision of the tumor was done. Histology confirmed features of atypical meningioma (WHO grade 2) with clear cell areas and invasion into the orbital tissues. Immunohistochemistry revealed strong epithelial membrane antigen (EMA) positivity and high Ki 67 index, which suggested increased possibility for recurrence. Post-operative course was uneventful and there were no further seizure episodes. Ocular movements recovered gradually. He was discharged two weeks after the surgery. Within four weeks of resection of the tumor, edema disappeared and serum albumin started to improve. At three months after surgery, urine albumin was negative, serum albumin was 4.1 gm/dl, and serum cholesterol was 170 mg/dl. Follow-up MRI scan three months after surgery showed mild gliotic changes [Figure 2]. Six months after surgery, he is doing well and his nephrotic state is in remission.
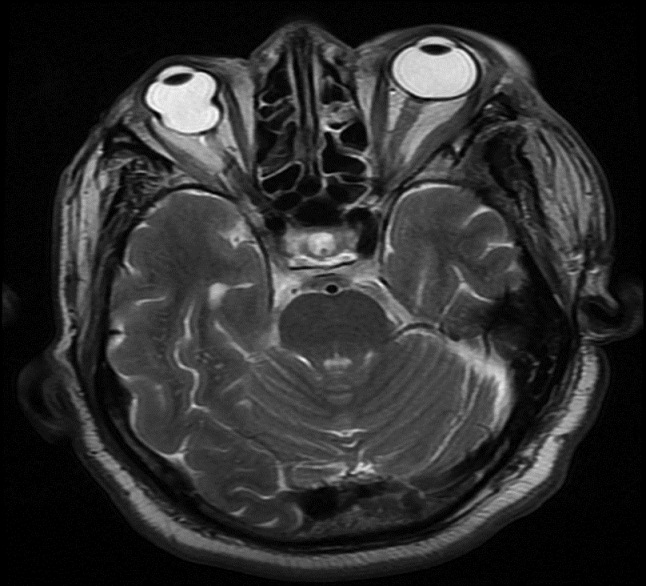
- Magnetic resonance imaging brain after surgery, showing no evidence of residual lesion or recurrence of meningioma
Discussion
Different types of glomerular diseases may be associated with tumors. Paraneoplastic nephrotic syndrome could be due to membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, or amyloidosis.[5] The most common association between solid tumor and nephrotic syndrome is that of membranous nephropathy, which also is the most common cause of adult-onset nephrotic syndrome [Tables 1 and 2]. It is characterized by the accumulation of immune deposits on the epimembranous aspect of the glomerular basement membrane in the absence of significant intraglomerular cellular proliferation. On the other hand, minimal change disease and focal segmental glomerulosclerosis are associated with hematological malignancies, most commonly Hodgkin's lymphoma and less often with non-Hodgkin's lymphoma, leukemia, plasma-cell disorders or thymoma.[6] Secretion of a toxic lymphokine by abnormal T cells may lead to glomerular injury in these disorders. In a small number of patients with malignancy, nephrotic syndrome occurs due to either AL or AA amyloidosis. The former is due to the deposition of fragments of monoclonal light chains, which may occur in association with multiple myeloma, whereas the latter reflects the deposition of fragments of the acute-phase reactant serum amyloid A protein, and has been described with a number of malignancies, particularly renal-cell carcinoma, Hodgkin's lymphoma and chronic lymphocytic leukemia.[6]


The temporal relationship between nephrotic syndrome and malignancy is variable: 40-50% of patients have symptomatic nephrotic syndrome before the diagnosis of cancer, whereas in 15-20% of the patients, the latter precedes the diagnosis of nephrotic syndrome. In the remaining patients, there is simultaneous presentation of both diseases. Membranous nephropathy in association with neoplasia is a well-known entity, especially in patients over 60 years of age. The incidence of malignancy-associated membranous nephropathy appears to increase with age, with peak incidence between 50 and 70 years of age.[47] Proteinuria occurs prior to or concomitantly with the diagnosis of the malignancy in over 80% of the cases. Approximately, 75% of the cases of membranous nephropathy in adults are idiopathic. Secondary membranous nephropathy has been recognized due to various other clinical conditions namely autoimmune diseases, infectious diseases, drugs, and tumors. Japanese investigators reported that glomerular staining for IgG1 was significantly stronger in malignancy- related membranous nephropathy and equal to IgG4, whereas in primary membranous nephropathy, there was predominant staining for IgG4. About 5-20% of the adults with membranous nephropathy have been reported to have a malignancy, most commonly a solid tumor-like carcinoma of the lung, kidney, breast, or gastrointestinal tract.[7]
Meningiomas are neoplasms which occur in the middle decades of life and account for 13-19% of all primary intracranial tumors. The majority are benign, slow growing, and well-circumscribed. Meningiomas in general, and especially those which belong to the atypical type, vary in clinical presentation and morphologic features making classification and prediction of recurrence difficult. In the revised edition of the WHO classification of tumors of the central nervous system,[8] three categories of meningiomas have been described – common (WHO grade 1), atypical (WHO grade 2), and anaplastic (WHO grade 3). Atypical meningiomas represent an intermediate category of tumor that has a higher relative risk for relapse. Patients with atypical meningiomas have improved long-term (10 years) survival rates compared to patients with anaplastic meningiomas (79% 10-year survival vs. 34.5%; however, 26% of recurring atypical meningiomas may assume a malignant phenotype.
Definitive treatment of paraneoplastic nephrotic syndrome would be to target the primary disease rather than the renal lesion. Until now, only isolated cases of remission of membranous nephropathy after treatment of the neoplasm have been reported, with a total of less than 20 cases related to tumors in the last 10 years.[4] Prognosis for patients who develop carcinoma and nephrotic syndrome has been generally poor.[9]
To the best of our knowledge, nephrotic syndrome associated with meningioma has not been described so far. We could not do a kidney biopsy as planned, because the patient had to be taken up for an urgent surgical excision of the tumor; following the surgery, he attained complete remission of nephrotic state, and hence a kidney biopsy was not done.
Conclusion
Although a convincing association between malignancy and glomerular disease was demonstrated in as early as 1966 by Lee,[2] nephrotic syndrome is a rare manifestation of tumor-associated paraneoplastic syndrome. Search for tumors is warranted in patients over the age of 55 years presenting with nephrotic syndrome. Paraneoplastic nephrotic syndrome has been reported to be associated with various solid malignancies, particularly adenocarcinomas of the colon and lung;[10] however, nephrotic syndrome has not been reported so far in association with a neurological tumor. Extensive review of the existing literature, indisputably suggests that this is probably the first time that paraneoplastic nephrotic syndrome is being described in a patient with a benign neurological tumor.[9] Remission of the nephrotic state occurred within 3 months after complete resection of the meningioma.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Long-term risk of cancer in membranous nephropathy patients. Am J Kidney Dis. 2007;50:396-403.
- [Google Scholar]
- The association of cancer and the nephrotic syndrome. Ann Intern Med. 1966;64:41-51.
- [Google Scholar]
- Development of features of glomerulopathy in tumor-bearing rats: A potential model for paraneoplastic glomerulopathy. Nephrol Dial Transplant. 2012;27:1786-92.
- [Google Scholar]
- Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70:1510-7.
- [Google Scholar]
- Glomerulonephritis and malignancy: A population-based analysis. Kidney Int. 2003;63:716-21.
- [Google Scholar]
- Nephrotic syndrome in chronic lymphocytic leukemia: A paraneoplastic syndrome? Clin Nephrol. 2000;54:492-7.
- [Google Scholar]
- Paraneoplastic glomerulopathies: New insights into an old entity. Kidney Int. 1999;56:355-77.
- [Google Scholar]
- Renal diseases associated with malignancies. Nephrol Dial Transplant. 2001;16:13-4.
- [Google Scholar]







