Translate this page into:
Renal transplantation across ABO barrier
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In India, patients without a compatible blood group donor are usually excluded from renal transplantation. For young patients, it is a difficult therapeutic choice to stay on long-term dialysis. We describe the case of a 19-year-old male patient who had blood group O +ve and had no compatible donor in the family. His mother was B +ve and was willing to donate. The patient had an initial anti-B antibody titer of 1:512 and underwent antibody depletion with plasmapheresis (11 sessions) and intravenous immunoglobulin (IVIG) 100 mg/kg after every plasmapheresis. He also received rituximab 500 mg for 3 days prior to transplant and was induced with basiliximab. At the time of transplant, his anti-B titers were <1:8. Post-operatively, he required four sessions of plasmapheresis and IVIG as his titers rebounded to 1:64. The titers then spontaneously subsided to <1:16 and have stayed at the same level for 6 months post-transplant. The patient continues to have normal renal function with a creatinine of 1.4 mg/dl% and has had no episodes of rejection.
Keywords
ABO incompatible
chronic kidney disease
renal transplant
Introduction
Kidney transplantation is the best treatment option for patients with chronic kidney disease Stage 5.[1] Paired kidney donation and ABO incompatible (ABOi) transplantation are the only options for patients with no compatible donors. Recent reports of ABOi transplant have shown comparable patient survival and acceptable long-term graft survivals.[23] We describe our first patient who had an ABOi transplant.
Case Report
A 19-year-old patient presented to our hospital with stage 5 CKD in May 2011. He was started on hemodialysis in another hospital and subsequently received 20 PRBC transfusions over 3 months.
The patient was seen in August 2011 and the option of kidney transplantation was discussed. The blood group of the patient was O +ve and no family member had the same group. The patient’s mother had blood group B. After discussing the pros and cons of ABO incompatible transplant, the mother was selected as the donor.
A flow-cytometry cross-match was negative. His baseline anti-B antibody titer was 1:512. He was planned for plasmapheresis (one plasma volume) in alternate days followed by intravenous immunoglobulin (IVIG) 100 mg/kg alternating with hemodialysis [Figure 1]. He was planned for induction with two doses of basiliximab (on days 0 and 4) and rituximab (500 mg, one dose) prior to transplantation and to transplant when anti-B titers were <1:8.
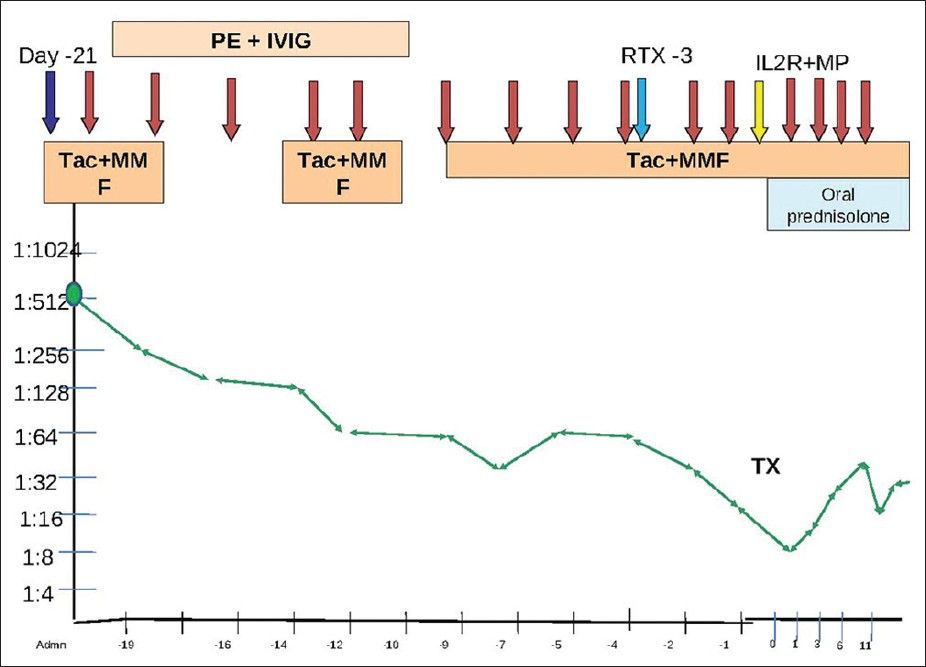
- The trend of Antibodies with immunosuppresion with time. PE: Plasmapheresis; IVIG: Intravenous immunoglobulin; Tac: Tacrolimus; MMF: Mycophenolate mofetil: TX: Date of transplant; RTX: Rituximab; IL2R: Interleukin-2 receptor antagonist Basiliximab; MP: Intravenous methylpredniolone Red arrows: Plasmapheresis; Blue arrow: Day of admission and start of treatment; Light blue arrow: Rituximab; Yellow arrow: Interleukin-2 receptor antagonist Basiliximab
He was started on tacrolimus (Tac) (0.1 mg/kg daily), mycophenolate mofetil (720 mg BD), and plasmapheresis/IVIG. He required 11 sessions of plasmapheresis and IVIG alternating with dialysis prior to transplant before his anti-B antibody titers decreased to <1:8. He developed severe hypertension for which Tac had to be stopped temporarily and restarted at half the dose once blood pressure had settled. On day 3, he was given a dose of rituximab 500 mg intravenously. He was transplanted on 2.11.2011 with pre-operative titers of 1:8. He was given induction with Inj. basiliximab 20 mg on days 0 and 4 and Inj. methylprednisolone 500 mg on day 0 followed by methylprednisolone 250 mg for 2 days and then oral prednisolone 40 mg which was tapered to 20 mg daily. Target blood levels of Tac in initial 3 months were 10–12 ng/ml and then 6-8 ng/ml. Mycophenolate mofetil (MMF) was reduced to 360 mg twice daily and prednisolone to 10 mg by 3 months.
Post-operatively, anti-B titers were monitored daily. He required four sessions of plasmapheresis and intravenous immunoglobulin on post operative days 1, 3, 6, and 10. Initially, his titers rose from 1:8 on POD 0 to 1:64 on POD 10 but then spontaneously declined to 1:16 thereafter. Post-operatively, he had a progressive decline in creatinine to 0.9 mg/dl. There was no episode of graft dysfunction/rejection. He was discharged on day 16.
Currently, he is 6-month post-transplant. He is on Tac 0.5 mg twice a day, MMF 360 mg BD, and prednisolone 10 mg OD. His creatinine is 1.4 mg/dl and anti-B titer is 1:16. A protocol biopsy at 3 months was normal and had mild C4d positivity.
Discussion
Initial attempts in transplanting across the ABO blood group were associated with high rates of early graft loss due to AMR and hence blood group compatibility became a pre-requisite for renal transplantation.[4] ABO blood group antigens are also expressed in the vascular endothelium, convoluted distal tubules, and collecting tubules.[5] The key mediators of antibody-mediated rejection are naturally occurring antibodies directed against ABO antigens.[6–8]
Takahashi et al.[2] published their experience of 441 patients in 9 years. Their protocol comprised of extracorporeal immunomodulation, pharmacological immunosuppression, splenectomy, and anti-coagulation. However, whether splenectomy is essential for successful ABOi KT remained unproven? Sonnenday et al.[9] found that the suppression of anti-ABO antibody after splenectomy was not significantly different from that of non-splenectomized patients and splenectomized recipients had a 25% greater mortality for 84 months.
Montgomery et al.[10] published their experience of 60 ABOi transplants and of these patients 32 patients had received splenectomy or rituximab or both, whereas 28 patients had no splenectomy and no rituximab. The outcomes of these two subgroups were comparable. Rituximab was administered on day 3 as the patient was intolerant to Tac and was only on MMF and his titers had bounced back.
Antibody depletion technique remains the cornerstone of ABOi and multiple techniques, such as plasma exchange, double-filtration plasmapheresis, and antigen-specific immunoadsorption are used to achieve ABOi. The main difference among these techniques is their degree of selectivity to achieve this.[11] However, none has been shown to be superior to other. The most common and least expensive method is therapeutic plasma exchange. This procedure eliminates approximately 20% of the anti-ABO antibodies with each session.
An important issue is isoagglutinin titer. Column agglutination by AutoVue method (ortho-clinical diagnostics) was utilized in this patient. In the USA, Koyabashi et al.[12] compared four antibody measurement techniques: The test tube technique, BioVue column agglutination technique, DiaMed-ID micro-typing system, and flow-cytometry. The comparison revealed that flow cytometry had the best reproducibility.
Another issue is the lack of consensus with regards to a "suitable" level of anti-A/B IgG or IgM titers on the day of transplantation. Tyden et al.[13] aimed to achieve IgG titer of <1:8 on the day of transplantation, whereas Tanabe et al.[14] had accepted an upper limit of 1:32 for IgG and IgM titers. Both studies reported good graft survival rates despite their differences.
Initial studies comparing ABOi subgroups showed that there was lower risk of adverse outcomes in patients with A2 blood group as compared to A1.[515] However, a recent study by Montgomery et al.[15] shows that among ABOi recipients, there was no statistically significant difference between A1 versus A2 donors and O versus non-O recipients.
Our patient did well and after 3 months, a protocol biopsy was done. Light microscopy was entirely normal; however, on immunofluorescence, C4d positivity was seen. This C4d positivity has been well described in ABOi transplants, the relevance of which is not clear. Ushigome et al.[16] have performed graft biopsies with 90 days of ABOi transplants and reported 85.7% of cases with ABOi transplants had C4d positivity with no features of AMR.
Conclusion
With better understanding of immunobiology, it has become possible to perform ABOi renal transplants. It is now clear that the short- and long-term outcomes of ABOi transplants are comparable to ABOc. However, it is unclear whether plasmapheresis or immunoadsorption, rATG or IL2RA, anti-CD20 or a combination is more effective or cost-effective. As ABOi Txp. becomes more widespread, randomized controlled trials answering the above questions are required.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089-96.
- [Google Scholar]
- Clinical outcome of ABO-incompatible living unrelated donor kidney transplantation. Urol Int. 2011;86:307-14.
- [Google Scholar]
- Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82:479-85.
- [Google Scholar]
- Antibody mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046-56.
- [Google Scholar]
- Consensus opinion from antibody working group on diagnosis, reporting, and risk assessment for antibody-mediated rejection and densensitization protocols. Transplantation. 2004;78:181-5.
- [Google Scholar]
- The role of ABO-incompatible living donors in kidney transplantation: State of the art. Semin Nephrol. 2007;27:408-13.
- [Google Scholar]
- Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315-22.
- [Google Scholar]
- ABO incompatible renal transplantation: A paradigm ready for broad implementation. Transplantation. 2009;87:1246-55.
- [Google Scholar]
- Isoagglutinin adsorption in ABO-incompatible transplantation. Transfus Apher Sci. 2010;43:231-5.
- [Google Scholar]
- A series of survey on assay for anti-A/B antibody by the Japanese ABO-incompatible Transplantation Committee. Xenotransplantation. 2006;13:136-40.
- [Google Scholar]
- Implementation of a protocol for ABO-incompatible kidney transplantation - A three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153-5.
- [Google Scholar]
- ABO-incompatible renal transplantation at Tokyo Women’s Medical University. Clin Transpl. 2003;17:175-81.
- [Google Scholar]
- Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603-9.
- [Google Scholar]
- Findings of graft biopsy specimens within 90 days after ABO blood group incompatible living donor kidney transplantation compared with ABO-identical and non-identical transplantation. Clin Transpl. 2010;24:16-21.
- [Google Scholar]







