Translate this page into:
Basics of kidney biopsy: A nephrologist's perspective
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The introduction of the kidney biopsy is one of the major events in the history of nephrology. Primary indications of kidney biopsy are glomerular hematuria/proteinuria with or without renal dysfunction and unexplained renal failure. Kidney biopsy is usually performed in prone position but in certain situations, supine and lateral positions may be required. Biopsy needles have changed with times from Vim–Silverman needle to Tru-cut needle to spring-loaded automatic gun. The procedure has also changed from blind bedside kidney biopsy to ultrasound marking to real-time ultrasound guidance to rarely computerized tomography guidance and laparoscopic and open biopsy. In very specific situations, transjugular kidney biopsy may be required. Most of the centers do kidney biopsy on short 1-day admission, whereas some take it as an outdoor procedure. For critical interpretation of kidney biopsy, adequate sample and clinical information are mandatory. Tissue needs to be stained with multiple stains for delineation of various components of kidney tissue. Many consider that electron microscopy (EM) is a must for all kidney biopsies, but facilities for EM are limited even in big centers. Sophisticated tests such as immunohistochemistry and in-situ hybridization are useful adjuncts for definitive diagnosis in certain situations.
Keywords
Biopsy needle
kidney biopsy
ultrasound
Introduction
The introduction of kidney biopsy is one of the major events in the history of nephrology. After unpublished attempts by Alwall in Sweden in 1944,[1] Brun and Iversen of Copenhagen in 1951[2] were the first to publish their experiences of aspiration biopsy with patients in the sitting position. However, the success rate in obtaining useful tissue remained low. It was Kark and Muehrcke in 1954[3] who performed the first kidney biopsy in the prone position using Vim–Silverman needle. Finally, in 1961, the publication of CIBA Foundation Symposium on Kidney Biopsy registered the coming of age of a clinically useful and acceptable technique.[4] Today most nephrologists prefer to use one of the spring-loaded, automatic or semiautomatic biopsy guns for kidney biopsy. The addition of ultrasonography and computerized tomography (CT) to locate the kidneys as an aid in positioning of biopsy needle has simplified the technique. As renal transplant is significantly different from native kidney biopsy, transplant biopsy is not being discussed in this review.
Indications of renal biopsy
Space prevents the listing of all indications of kidney biopsy, but for all practical purposes, the following are broad indications:
-
Unexplained acute or rapidly progressive renal failure
-
Nephrotic syndrome and significant non-nephrotic proteinuria
-
Persistent glomerular hematuria
-
Systemic diseases with renal involvement
-
Renal allograft dysfunction.
All these indications are not absolute. In each situation, if associated clinical and laboratory investigation suggest a predictable histological pattern, kidney biopsy may not be required.
Following are the contraindications of kidney biopsy:
Absolute
-
Small kidneys
-
Abnormal coagulopathy
-
Uncontrolled hypertension.
Relative
-
Solitary kidney
-
Uncooperative patient
-
Unable to lie flat on bed
Renal Biopsy Technique
Position of the patient
Kidney biopsy is usually performed in the prone position. Lower pole of the left kidney is preferred to reduce the risk of inadvertent injury to a major vessel. However, if the patient is obese or has breathing difficulty, then a supine anterolateral position (SALP) may be more suitable. In a recent study, percutaneous ultrasound-guided kidney biopsy was performed in the SALP in obese patients with greater comfort and less breathing difficulty than in the prone position, with no reduction in diagnostic yield or increase in complications.[5]
Biopsy instruments
The era of biopsy instruments has evolved from Vim–Silverman needle to manually operated sheathed needle (Tru-Cut) and now to automatic spring-loaded biopsy guns [Figures 1 and 2]. Automatic biopsy guns perform better on direct comparisons. In a randomized prospective study comparing Tru-Cut and automated biopsy guns, both techniques gave adequate tissue samples, but bleeding was more with Tru-Cut needle.[6] In another retrospective analysis including 64 blind biopsies, 16 gauge Tru-Cut in 56 and Silverman needle in 8 were compared with 65 ultrasound guided 18-gauge (full-core) spring-loaded biopsy gun. The need for re-biopsy, large hematomas, post-procedure vascular intervention, and fall in hemoglobin over next 24 h all were significantly more with blind biopsy technique.[7]
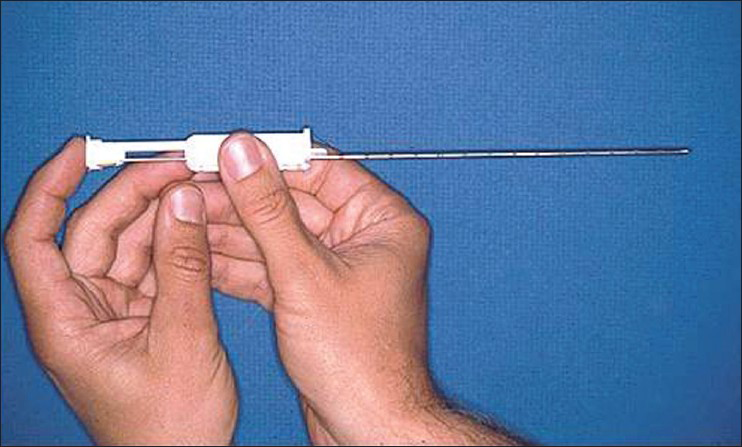
- Tru-Cut biopsy needle

- Automatic spring-loaded biopsy gun
Biopsy guiding
Currently, biopsies are usually performed after ultrasound marking or under real-time, ultrasound-(US) guidance. US guided automated biopsy guns have a failure rate of 0-3%, and complications in 0-7% with major complications in ≤3% of patients. The need for surgical interventions ranged from 0 to 0.8%.[8910]
CT-guided approach can be better employed in high-risk and obese individuals. In one study, 25 biopsies were performed using CT fluoroscopic imaging. In all cases, the coaxial trocar was easily placed, and adequate tissue was obtained after only two passes in 19/22 patients, whereas 3/22 patients had to be re-biopsied using the same technique in order to obtain better specimens. Small hematomas were detected by CT scan in all patients. Only one patient required transfusion. The ability to visualize kidneys accurately during and after biopsy represents a distinct advantage over other current biopsy techniques.[11]
Other Biopsy Accesses
Laparoscopic kidney biopsy
With improvements in efficacy and safety, conditions such as solitary kidney and obesity that were assumed absolute are now considered relative contraindications of percutaneous biopsy. However, there are some patients in whom a percutaneous approach is associated with unacceptable risk. In these cases, kidney biopsy under direct vision is a reliable option. Kidney biopsy under direct vision can be performed with an open incision or laparoscopically. This method allows positive identification of kidneys for a macroscopic diagnosis, and biopsy and homeostasis are better achieved under direct view. The possible indications for laparoscopic kidney biopsy include the following:
-
Failed percutaneous biopsy
-
Chronic anticoagulation state/coagulopathy
-
Morbid obesity
-
Solitary kidney
-
Multiple bilateral kidney cysts
-
Kidney artery aneurysm
-
Uncontrolled hypertension
Procedure
The biopsy is performed under general anesthesia with the patient in the lateral decubitus position. A two-port technique is used: a 10-mm laparoscopic port is placed above the iliac crest in posterior axillary line and a 5-mm port is placed at the same level in the anterior axillary line. The lower pole of kidney is exposed after minimal blunt retroperitoneal dissection. Laparoscopic cup biopsy forceps are used to take multiple superficial cortical biopsies [Figure 3]. The biopsy site can be fulgrated with argon beam coagulator and a sheet of oxidized cellulose can be applied there upon.[1213]

- Laparoscopic cup biopsy forceps
The advantages of laparoscopic biopsy as against open biopsy are as follows:
-
Outdoor basis
-
Under vision
-
Wound infection is less compared to that in open kidney biopsy
-
Adequate homeostasis achieved
-
Safe and reliable
-
If required, prompt conversion to open procedure can be made.
The disadvantages as against closed percutaneous biopsy are as follows:
-
Costly
-
Requires general anesthesia
-
More invasive than closed percutaneous biopsy.
Transjugular kidney biopsy
The transjugular route has been used for thousands of liver biopsies since its description in 1964. Even when used for severely ill patients, morbidity and mortality rates were extremely low, partly because when hemorrhage occurs the blood reenters the circulation. In 1989, Fre′de′ric Mal did a liver biopsy, which the pathologist reported as kidney tissue. This led to the idea of transjugular kidney biopsy. The indications are similar to those of laparoscopic kidney biopsy. Additionally, simultaneous liver–kidney biopsy can be performed, especially in disorders involving both liver and the kidney.
Procedure
Transjugular kidney biopsy is performed in an angiography suite. An internal jugular vein puncture is performed under ultrasound guidance. The right side is preferential so as to allow more direct access to the inferior vena cava (IVC) for the biopsy needle. The sheath is then advanced over a stiff guide wire into the IVC under fluoroscopic guidance. The kidney vein is selectively catheterized using a 4-F or 5-F catheter introduced through the sheath. The sheath is then advanced over the catheter into the kidney vein and an optimal peripheral position located with the aid of contrast enhancement. Biopsy needle is then inserted and tissue sample is obtained with the aid of spring-loaded gun. Once the needle containing the tissue specimen had been removed, contrast can be injected to identify capsular perforation, and embolization coils may be placed at the discretion of the operator.[14]
The following are relative advantages of transjugular biopsy:
-
Safer as needle passes into vein and away from major vessels. Any bleed directs to vein
-
Capsular perforation managed with elective coil embolization.
The following are the disadvantages:
-
Arterio-calyceal bleed.
Post-Kidney Biopsy Care
Following kidney biopsy, vitals are checked at frequent intervals for initial few hours. Bed rest is advised for initial 8-10 h. In some centers, kidney biopsy is performed as an outpatient procedure, but in majority of centers, it is an inpatient procedure. Routine post-biopsy ultrasound is not recommended. The common complications are local pain, minor bleeding in urinary tract, perinephric hematoma, and uncommonly arteriovenous fistula.
Adequacy of Tissue Sampling
Sample size – two cylinders with a minimal length of 1 cm and a diameter of at least 1.2 mm are needed.
-
Needle gauge: 18 gauge (G).
-
Number of glomeruli for adequate diagnosis:
-
For glomerular lesions: 5.
-
For tubulointerstitial lesions: 6-10.
-
For transplant kidney: 7.
Clinical Information and Transportation
Kidney biopsy should be accompanied by adequate clinical information to enable proper interpretation of findings. Statement that “one cannot feed in garbage and get out fruit juice” is most appropriate while providing information to the pathologist. The biopsy specimen must be handled gently when removed from the biopsy needle; an 18G needle or a thin wooden stick, for example, is ideal. Normal saline can also be used to wash the sample off the needle. The use of a “dissecting microscope” can be of assistance in assessing sample adequacy. Another alternative is the use of a standard light microscope. The tissue is placed on a glass slide with normal saline and examined with or without a cover slip on a wet mount. A trained observer can recognize fat, skeletal muscle, and other non-kidney tissue. Knowledge of the glomerular content of the sample can also guide division of tissue for the various test modalities.
The standard approach is to first procure tissue for electron microscopy (EM) from each core by removing 1 mm cubes from the ends and placing them in cooled glutaraldehyde or other fixative suitable for EM [Figure 4]. Some clinicians prefer that the pathology laboratory obtain tissue for EM from the ends of the formalin-fixed tissue. If the specimen is to be sent to a laboratory that uses immunofluorescence (IF), the first core can be cut in half by cross-sectioning and the larger piece placed in formalin or another fixative suitable for light microscopy (LM); the smaller portion is saved for IF evaluation. If a second core is obtained, the ends should be taken for EM and the specimen again divided almost in half, with the larger tissue core now kept for IF and the smaller for LM.[15]

- Diagram to illustrate division of kidney biopsy cores in the absence of a dissecting microscope for laboratories using immunofluorescence
Sectioning and Fixation
The tissue sections should be no greater than 2-3 μm in thickness, as the definition of glomerular pathology, especially regarding cellularity, is dependent on this thickness. Sectioning should include LM, IF, and EM. Thin sections are important for LM and silver methenamine staining as they reveal fine structural and cellular details, which are not possible in thick section. Serial sections of 2-μm thickness are cut and at least two sections should be placed on each slide. Good histology also demands good fixation. If fixation is delayed and imperfect, it cannot be improved later. The most commonly used fixative for LM is buffered, 10% aqueous formaldehyde solution (formalin). Formalin is stable at room temperature, provides acceptable morphology, and allows immunohistochemistry (IHC) or molecular studies to be performed. Some laboratories prefer alcoholic Bouin's, Duboscq–Brasil, or Zenker's fixatives that provide better preservation of certain morphologic details.
Staining and Light Microscopy
For LM, the elucidation of lesions of glomeruli mandates that a variety of histochemical stains be used and that tissue sections be cut thinner than for other tissues. For the elucidation of glomerular structure and pathology, it is necessary that the extracellular matrix components (basement membrane and mesangial matrix) be preferentially stained. In paraffin-embedded sections, hematoxylin and eosin stain does not ordinarily allow for distinction of extracellular matrix from cytoplasm in a clear or convincing manner. Periodic acid-Schiff (PAS), periodic acid-methenamine silver (Jones), and Masson's trichrome stains all provide excellent definition of extracellular material. A variety of common histochemical stains used to evaluate kidney biopsy are shown in Table 1.

Immunofluorescence
Immunofluorescence is best performed on unfixed, frozen sections. Tissue can be transported to the laboratory fresh on saline-soaked gauze or in Michel's fixative. Serial sections are cut at 2-4 μm in a cryostat. Fluorescein-labeled antibodies used for the antigens that should be routinely examined include immunoglobulins (primarily IgG, IgM, and IgA), complement components (primarily C3, C1q, and C4), fibrin, and kappa and lambda light chains. Additional antibodies may be required in specific circumstances, for example, amyloid typing, collagen IV alpha chains in hereditary nephritis, IgG subclasses, virus identification, lymphocyte phenotyping in allografts in suspected cases of post-transplant lymphoproliferative disorder (PTLD), and C4d in renal allograft biopsies.
Electron Microscopy
The tissue for EM may be fixed in 2-3% glutaraldehyde or 1-4% paraformaldehyde. Adequate fixation can also be obtained when tissue is fixed in buffered formalin. EM cannot be performed on tissues exposed to mercury-based fixatives (e.g., Zenker's). Rapid placement of the sample into the fixative will provide the best outcome. Tissue can be reprocessed from the paraffin or the frozen block if no glomeruli are available in the EM sample. However, such reprocessed tissue will have poor morphologic preservation. Toluidine blue-stained 1-μm thick sections are examined to identify appropriate structures for thin sectioning and examination with the electron microscope. In general, one or two glomeruli are examined ultrastructurally. For glomerular and some tubulointerstitial diseases, this method is mandatory and helps localize deposits, detects extremely small deposits, and documents alterations of cellular and basement membrane structure. IF and EM are also often necessary and helpful in diagnosing other tubular, interstitial, and vascular lesions.
EM is most helpful in the following clinical situations:
-
Hematuria, especially microscopic, with or without proteinuria
-
When there is a family history of renal disease.
-
When there is a symptomatic proteinuria, with normal renal excretory function.
Immunohistochemistry
IHC detects specific proteins by mono-or polyclonal antibodies raised against that protein in biopsy. Some of the examples for such proteins are:
-
Hepatitis B virus and SV40 antigen for BK Polyoma virus infection.
In-situ Hybridization
ISH uses labeled cDNA or RNA probes. It localizes specific DNA/RNA sequence in tissue section which is then quantitated using autoradiography or fluorescence microscopy. The commonly used ones are as follows:
-
BK virus.
-
EB virus probes in the diagnosis of PTLD.
-
Pathogenic cytokines such as platelet-derived growth factor, epithelial growth factor, etc.
Tissue Examination and Interpretation
Under the microscope, first a low-power screening examination of the specimen should be carried out. This will give an idea of area of defect and will also help in localizing that the defect is in glomerulus, tubule, and interstitium, and/or blood vessels.[16] In addition to the site of lesions, the distribution of lesion is also important from the pathology point of view.
-
Diffuse change: Changes occurring in all the glomeruli.
-
Focal changes: Changes occurring in few glomeruli only.
-
Global changes: Whole glomerulus is involved.
-
Segmental changes: Only some part of glomerulus is involved.
The next issue is to categorize whether the lesion is active or chronic type. Some of the examples of active and chronic lesions are shown in Table 2.

Some of the common lesions seen in kidney biopsy and their examples are as follows:
Abnormalities in Glomerular Capsule
Glomerular capsule is made up of outer basement membrane and inner epithelium. Between glomerular capsule and visceral epithelial cell layer is capsular space. Abnormalities can be in basement membrane, epithelium, and capsular space. Common abnormalities, their causes, and associated findings in relation to glomerular capsule are shown in Table 3 and in glomerular basement membrane in Table 4.


Cellular Proliferation
There are three types of cellular elements in glomerulus: Endothelial, epithelial, and mesangial cells. Endothelial cells line the capillary from inside. It has small nucleus, dense chromatin, and very little cytoplasm. Epithelial cells line the capillary from outside. There is a large nucleus, chromatin is loose and indistinct, and cytoplasm is copious. Mesangial cell resembles endothelial cells. Mesangial cell is PAS positive and nucleus is darkest compared to all other cells. Grossly, endothelial cell comprise 45%, mesangial cells 25%, and epithelial cells 30% of all cellular elements of glomerulus. Crescents represent accumulation of cells and extracellular material in the urinary space. This is associated with proliferation of visceral and perhaps parietal epithelial cells and accumulation of monocytes and other blood cells in the urinary space. The cellular composition of the crescent varies depending on the type of disease. Crescents most commonly heal by organization (scar formation). With an admixture of cells and collagen, the crescent may be cellular, fibrocellular, or fibrous. Common cellular abnormalities are shown in Table 5.

Peripheral migration and interposition of mesangium: Mesangial cells and often matrix extend from the central lobular portion of the tuft into the peripheral capillary wall, migrating between endothelial cell and basement membrane and causing capillary wall thickening with two layers of extracellular matrix. This two-layer or double-contour appearance may involve a few or all capillaries.
Alteration in visceral epithelial cell morphology: This abnormality requires the EM to detect. In association with protein loss across the glomerular capillary wall, the epithelial cells change shape; the foot processes retract and swell, resulting in loss of individual foot processes and a near solid mass of cytoplasm covering the glomerular basement membrane. This loss or “effacement of foot processes” is also incorrectly known as fusion because it was initially thought that adjacent foot processes fused with one another.
Increase in extracellular matrix implies an increase in mesangial matrix or basement membrane material. In the former instance, this may be in a uniform and diffuse pattern in all lobules or cause a nodular appearance to the mesangium. Increased basement membrane material takes the form of thickened basement membranes, an abnormality that is best appreciated by EM.
Healed Lesions
With aging of the lesion, although the cellularity decreases or disappears, PAS positivity persists. So, healed proliferative lesions are PAS positive and the term “sclerosis” or scar is often used. It leads to obliteration of capillaries and solidification of all or part of the tufts. Sclerosis may be associated with obliteration of the urinary space by collagen along with increased extracellular matrix in the capillary tufts. When the entire glomerulus is involved, this is known as complete sclerosis; an older and less precise term is glomerular hyalinization. As against this, necrotizing lesions are PAS negative and the healed necrotizing lesions result in “fibrosis,” which is PAS negative.
Vascular Lesions
Afferent arteriole is made up of smooth muscle and is lined by endothelium, which is continuous with that of glomerulus. Efferent arteriole is smaller than afferent arteriole in outer cortex and is equal or larger in juxtamedullary glomerulus. Afferent arteriole is recognized against efferent arterioles by the proximity to interlobular artery, more conspicuous muscular coat, and lumen is usually not filled with RBC. As both the arterioles are in close proximity and continuity to glomerulus, they should be considered as one structure. In general, the renal arteries and arterioles respond to injuries in a manner similar to other vascular beds. However, the kidneys are more frequent targets of vascular injury because of their high blood flow.
The major lesions affecting renal vasculature include the following:
-
Thrombosis
-
Fibrin deposition in the walls of arteries, arterioles, and glomerular capillaries;
-
Inflammation and necrosis of vascular walls; and
-
Arteriosclerosis.
Fibrinoid change
Fibrinoid material is homogenous, refractile, eosinophilic, often granular with poorly defined edge, and is PAS negative. If there is only the presence of fibrinoid material, it is called fibrinoid change. In fibrinoid necrosis, in addition to fibrinoid material, there is inflammation with infiltration of mononuclear cells and neutrophils, swelling of endothelial cells, presence of pyknotic nuclei/karyorrhexis, and disruption of elastic lamina.
Hyaline change
Presence of hyaline in tissue is referred to as hyaline change. Hyaline is PAS positive, acellular, homogenous, refractile, less eosinophilic material than fibrinoid material, and its boundaries are better defined than fibrinoid. It is stained with van Gieson stain. Table 6 shows common vascular lesions.

Tubulointerstitial Lesions
Tubular cells may exhibit a variety of degenerative changes, or may undergo acute reversible and irreversible damage (necrosis). The degenerative lesions are often in the form of intracellular accumulations. For example, lipid inclusions in proximal and less commonly, distal tubular cells result from hyperlipidemia and lipiduria of nephrotic syndrome, and protein reabsorption droplets (“hyaline droplets”) accumulate in proximal tubular cells in association with albuminuria and its reabsorption by tubular epithelium. Additional locally induced abnormalities include uniform fine cytoplasmic vacuolization consequent to hypertonic solution infusion (e.g., mannitol and sucrose). Tubular cells may be sites of “storage” of hemosiderin in patients with chronic intravascular hemolysis, high iron load, or glomerular hematuria. Few metabolic storage diseases affect tubular epithelium; among others are cystinosis with crystals and glycogen storage diseases and diabetes mellitus with abundant intracellular glycogen. Vacuoles, especially large and irregular, may be associated with hypokalemia.
On the contrary, reversible and irreversible changes are features of acute tubular necrosis. These include loss of brush border staining for proximal cells, diffuse flattening of cells with resulting dilatation of lumina, loss of individual lining cells, and sloughing of cells into lumina. Manifestations of repair or regeneration include cytoplasmic basophilia and mitotic figures.
The morphologic features of atrophy of tubules include not only diminution in caliber, but also more importantly irregular thickening and wrinkling of basement membranes. Adjacent tubules are invariably separated from one another in this circumstance. The intervening interstitium is almost always fibrotic, with or without accompanying inflammation. Other structural forms of tubular atrophy include uniform flattening of cells, hyaline casts in dilated lumina, and close approximation of tubules, resulting in a thyroid-like appearance to the parenchyma. Common tubular abnormalities are shown in Table 7.

Interstitial Lesions
There are limited structural abnormalities in interstitial injury. Commonly found changes are edema, inflammation, and fibrosis. Both edema and fibrosis are associated with separation of normally closely apposed tubules. With interstitial edema only, the basement membranes of tubules are of normal thickness and contour. In contrast, with fibrosis the tubules are invariably atrophied with thickened and irregularly contoured basement membranes. The distinction between an acute and a chronic interstitial process is made based on the presence of edema (acute) or fibrosis (chronic), regardless of the character of any infiltrating leukocytes. With interstitial inflammation, especially when acute, the leukocytes, which gain access to the interstitium from the peritubular capillaries, usually extend into the walls of tubules. During this process, there may be damage to and destruction of tubular basement membranes as well as degeneration of epithelial cells. This often results in spillage of tubular contents into the interstitium.
The type(s) of cells in an interstitial inflammation depend(s) on the nature of the inflammatory process. Polymorphonuclear leukocytes, as expected, are present in early phases of bacterial infections; however, they are usually replaced by lymphocytes, plasma cells, and monocytes approximately 7-10 days following the onset of infection. On the contrary, other infectious agents may elicit only a “round cell” response. Cell-mediated forms of acute inflammation, even in very early stages, are characterized by lymphocytic infiltrate, with or without plasma cells, monocytes, and granulomata.
Besides inflammatory cells, the interstitium may contain abnormal extracellular material such as amyloid, immunoglobulin light chains, immune complex deposits, etc., This may be in association with similar infiltrates in glomeruli, or less commonly, may be restricted to the interstitium.
Conclusion
Kidney biopsy is an indispensable tool for current practice of evidence-based medicine. The clinicopathology correlation is a great challenge for both pathologists and nephrologists. LM, IF, and EM should be done routinely in all biopsies. Kidney biopsy, appropriately processed and interpreted, will yield the correct clinicopathologic diagnosis, leading to the appropriate therapeutic strategy while, at the same time, providing key prognostic information.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Aspiration biopsy of the kidney, including i.a. A report of a case of amyloidosis diagnosed through aspiration biopsy of the kidney in 1944 and investigated at an autopsy in 1950. Acta Med Scand. 1952;143:430-5.
- [Google Scholar]
- A CIBA Foundation Symposium on Renal Biopsy. Clinical and Pathological Significance. London: Churchill; 1961.
- [Google Scholar]
- Percutaneous ultrasound-guided renal biopsy in supine antero-lateral position: A new approach for obese and non-obese patients. Nephrol Dial Transplant. 2008;23:971-6.
- [Google Scholar]
- A randomized, prospective, comparative study of manual and automated renal biopsies. Am J Kidney Dis. 1998;32:426-31.
- [Google Scholar]
- Percutaneous renal biopsy: Comparison of blind and real-time ultrasound-guided technique. Semin Dial. 2007;20:355-8.
- [Google Scholar]
- Safety of ultrasound-guided percutaneous renal biopsy-retrospective analysis of 1090 consecutive cases. Nephrol Dial Transplant. 1998;13:975-7.
- [Google Scholar]
- Yield and complications in percutaneous renal biopsy. A comparison between ultrasound-guided gun-biopsy and manual techniques in native and transplant kidneys. Acta Radiol. 1997;38:431-6.
- [Google Scholar]
- Percutaneous native renal biopsy: Comparison of a 1.2-mm spring-driven system with a traditional 2-mm hand-driven system. Am J Kidney Dis. 1994;23:498-503.
- [Google Scholar]
- CT-guided kidney biopsy: A new, safe and effective technique (Abstract) J Am Soc Nephrol. 2001;12:738.
- [Google Scholar]
- Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555-63.
- [Google Scholar]







