Translate this page into:
Effect of sickle cell crises on glomerular filtration rate in children with sickle cell disease in Ilorin, Nigeria
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
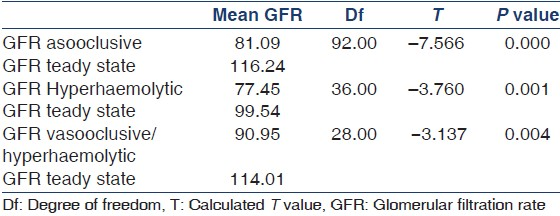
Children with sickle cell disease (SCD) are plagued with incessant crises. There are few studies on the effect of sickle cell crises on renal function as determined by the glomerular filtration rate (GFR). This study was done to assess the effect of sickle cell crises on GFR during crises and after recovery into the steady state. GFR was assessed using the formula derived by Schwartz et al., for consecutive SCD patients aged between 3 and 18 years who came in crises and after recovery into the steady state. A total of 81 patients with a mean age of 9.95 ± 4.15 years in 81 episodes of crises met the inclusion criteria. Majority of the children (47) had vasooclusive crises, 19 had hyperhaemolytic crises, and 15 had features of both vasooclusive and hyperhaemolytic crises. The means value of GFR in ml/min/1.73 m2 rose from 81.09 ± 22.92 to 116.24 ± 22.11 subsequent to recovery from vasooclusive crises into the steady state, from 77.45 ± 18.48 to 99.54 ± 17.71 following recovery from hyperhaemolytic crises into the steady state and from 90.95 ± 17.53 to 114.01 ± 22.44 following recovery from crises with features of both vasooclusive and hyperhaemolytic crises with corresponding significant P values of 0.000, 0.001, and 0.004 respectively. The reduced GFR observed during vasooclusive and hyperhaemolytic crises improved significantly following recovery into the steady state.
Keywords
Glomerular filtration rate
schwartz formula
sickle cell crises
sickle cell disease
Introduction
Sickle cell disease (SCD) is a common health problem in Nigerian children with an incidence of the homozygous sickle cell anemia (Hb SS) and the heterozygous, sickle cell hemoglobin C disease (Hb SC) being 1.5% and 1.3% respectively.[12] Compound heterozygosity with β+ -thalassemia (Sβ+ -thalassemia) is, however, rare in Nigeria.[3] Whereas infection is the leading cause of death among children with sickle cell anemia, deaths from chronic renal failure take prominence after the first three decades of life.[45] However, with improvement of knowledge of medical management of the disease and better living standards, a longer survival is expected among these children and hence the risk of death from renal failure may become more frequently encountered.
In Africa, few studies on the effect of SCD on renal function as determined by the glomerular filtration rate (GFR) have been performed with conflicting results.[6–10] The present study, therefore, aims at studying the effects of sickle cell crises on GFR among children seen at the University of Ilorin Teaching Hospital, Ilorin, Central Nigeria and thereby add to bridge the information gaps.
Materials and Methods
This prospective study was carried out between May and November 2004 at the University of Ilorin Teaching Hospital, Ilorin, Nigeria, after obtaining clearance from the Hospital’s Ethical Committee. An informed written consent was obtained from the subjects and parent/legal care. The subjects were recruited consecutively as they presented in crises at the Emergency Pediatric Unit of the Hospital, and they served as their own controls following recovery into the steady state. Care of the patients in vasooclusive crises (VOC) included rehydration until normal hydration was established and pain had been relieved. Close monitoring of vital signs during rehydration was done to prevent cardiac failure from fluid overload. Doses and choice of analgesics were individualized to achieve sufficient pain relief, i.e., dihydrocodeine and/or pentazocine were prescribed initially, and pethidine was also administered to patients whose pain was not relieved on the initial two analgesics. Pentazocine and pethidine were given in adequate doses and in a fixed schedule at regular intervals enough to suppress and prevent pain and dependence. Antimalarial chemotherapy, including Chloroquine and Artemisinin-based combination were administered for uncomplicated malaria. Quinine (intravenous or oral) and intramuscular artemether were administered to treat subjects with severe malaria. Empiric antibiotics against Streptococcus pneumoniae and Salmonella were given when fever persisted for 72 h subsequent to adequate antimalarial therapy and before blood culture and sensitivity results became available. For hyperhemolytic crises, severe anemia was corrected by blood transfusion (with blood group AA) as whole blood given at 20 ml/kg body weight over four hours without frusemide in the presence of clear evidence of hypovolemia and packed cells at 15 ml/kg body weight over four hours with frusemide in the absence of hypovolemia. Oxygen was provided as needed. Treatment for malaria and empiric antibiotics were given as discussed earlier. When patients recovered into the steady state, routine drugs, including folic acid and proguanil were regularly administered as chemoprophylaxis for anemia and malaria respectively.
Inclusion criteria
Patients with hemoglobin SS, and SC as confirmed by electrophoresis using cellulose acetate paper, age range between 3 and 18 years.
-
In a steady state[11] when they satisfied the following criteria
-
Absence of fever at presentation and for 4 weeks after the last crisis
-
Absence of skeletal and and/or abdominal pain at presentation and within the 4 weeks after the last crisis.
-
Otherwise active and going about their routine daily activities.
-
-
In a clinically defined state of
-
Vasooclusive crisis[12] (VOC) - complaints of skeletal and/or abdominal pains without clinical or radiological evidence of osteomyelitis or surgical abdomen
-
Hyperhemolytic crisis[13] - a recent recurrence or deepening of jaundice in association with reticulocytosis (Reticulocyte Index [RI] ≥1%) and a fall of the packed cell volume (PCV) from the steady state if known or with PCV ≤ 15%
-
Acute sequestration crisis[13] - a rapid increase in spleen size with reticulocytosis (RI ≥ 1%) and no jaundice or recent splenic enlargement and with PCV ≤ 15%
-
Aplastic crisis[14] - a severe anemia (PCV ≤ 15%) associated with reticulocytopenia (RI < 1%)
-
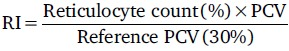
-
Exclusion criteria
-
Subjects less than three years because of known variation of GFR in children below this age and the drastic variability of serum creatinine (Scr) with increasing age up to the age of 3 years;[15]
-
Subjects with a known renal disease
-
Subjects who were initially healthy but subsequently developed renal disease during follow-up.
For each recruited patient, the GFR in mls/min/1.73 m2 during a crisis, and steady state were calculated using the Schwartz formula. Only method by Schwartz et al. was utilized.
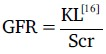
Where K = constant of proportionality
= 0.55 for children and adolescent girls (13-21 years of age)
= 0.70 for adolescent boys (13-21 years of age).
L = body height in cm.
Scr = Serum creatinine in mg/dl.
Sample for Scr and PCV estimation was collected (4 ml into a plain ethylene diamine tetra-acetic acid EDTA bottle) before the commencement of treatment for the crises (i.e., before intravenous fluid administration, analgesics etc.) Estimation of Scr was done using the Jaffe’s method on Corning colorimeter reading at 520 nanometers[17] Validation of Scr measurement was maintained strictly for coefficient of variation of ± 2% for within-run samples and ± 4% for between-days samples. PCV was carried out by the author at the emergency pediatric unit. The capillary tubes were spun using the centrifuge machine running at 2500 revolutions per minute for 5 min. The height was measured by a standard method to the last completed 0.1 cm. After appropriate verification, the data were analyzed using the Epi-info software Package (Version 6 of 1999) and SPSS version 11.1. Student’s t-test for comparing the mean of two samples (paired ’t’ test) was used to test for significance of the differences between means of GFR in crises and in the steady state. The level of significance was set at values of P < 0.05.
Results
A total of 102 patients were seen in 102 crises, but 81 were followed-up into the steady state. Twenty-one could not enter the study for various reasons. These included 12 patients who were under age of 3 years; five others with VOC were lost to follow up. Four died before they could be transfused with blood. Out of the 81 patients who were followed-up into the steady state, 47 (58%) had VOC, 19 (23.4%) presented with hyperhemolytic crises and 15 (18.6%) presented with features of both VOC and hyperhemolytic crises. The age group, gender, and genotype distribution of the study population is as shown in Table 1. The means value of GFR (ml/min/1.73 m2 ) rose from 81.09 ± 22.92 to 116.24 ± 22.11 following recovery from VOC into the steady state, from 77.45 ± 18.48 to 99.54 ± 17.71 following recovery from hyperhemolytic crises into the steady state and from 90.95 ± 17.53 to 114.01 ± 22.44 following recovery from crises with features of both vasooclusive and hyperhemolytic crises with corresponding significant P values of 0.000, 0.001, and 0.004 respectively. The comparison of mean glomerular filtration rate during crises and in the steady state is as shown in Table 2.

Discussion
The significant improvement of GFR following recovery into the steady state of the subjects with VOC was in agreement with the earlier works of Addae[7] and Aderibigbe,[8] who studied the effects of VOC in children and adult patients with heterogeneous HbSS and HbSC population using mannitol and 24-h urinary creatinine clearance respectively. The agreement of the current study and their findings is even more impressive, considering the fact that different methods of GFR assessment were employed, and this study was carried out exclusively in the pediatric age group.
However, the reduction of the GFR observed in VOC in this study differed from the results of Ocheke,[9] who observed increased GFR among children and that of Kenotey-Ahulu,[10] who observed same among children and adult’s population with VOC. While it may be that the hitherto recognized unreliability of collecting 24-h urine for creatinine clearance among pediatric population may have contributed to this observed increase in GFR, the administration of increased intravenous fluid imperative for standard management of patients during VOC, may also have contributed to this increase via expansion of the intravascular volume during the 24-h urine collection for creatinine clearance as it is done traditionally. Aderibigbe et al.[8] however, did not find increased GFR in their own studies using similar methods of 24-h urine clearance and their patients also received increased intravenous fluid.
The method (Schwartz formula[16]) employed by the current study has eliminated the potential contribution of intravascular volume expansion on increment in GFR as blood for Scr estimation was drawn promptly on arrival before the commencement of fluid therapy.
The reduced GFR observed during VOC in this study, and those of others[78] may be attribu to glomerular microvascular occlusion by sickled erythrocytes and several other events, which are well known to occur during VOC.[18–23] Furthermore, the stress and pain associated with vasooclusive crisis may give rise to an increase in the sympathetic discharge and a rise in the level of blood anti-diuretic hormone and adrenalin (causing mesangial cells contraction) with a resultant decrease in the GFR.[2425] The cumulative effect of all these factors (glomerular occlusion and contraction of mesangial cells) is a reduction in the effective surface area available for filtration and hence the reduced GFR observed during VOC. A search into the literature did not show that much work had been published on the effect of hyperhemolytic crises on the GFR of children with the SCD even though hyperhemolytic crises have been identified as a major cause of hospitalizations in one study.[26] However, the major pathogenetic mechanism operating in hyperhemolytic crises is that of acute exacerbation of hemolysis on chronic hemolytic process resulting in more severe anemia than what is obtained in the steady state. This study showed that there was an observed reduction in the mean GFR during hyperhemolytic crises with a statistically significant improvement in the mean GFR following recovery into the steady state. Although circulatory adjustments to anemia had increased the cardiac output and increased renal blood flow (i.e., increased GFR), blood is eventually diverted away from the kidney to other organs like the heart, the brain, and the adrenals which are more susceptible to hypoxia.[27] Consequently, the hypoxic injury of the acute anemic state may cause glomerular endothelial damage with a resultant reduction in the effective filtration surface area and hence the observed reduction in GFR as found in the present study.
The current study would also sum to indicate that sickle cell anemia patients who were afflicted with the combination of hyperhemolytic and VOC tend to have glomerular dysfunction, which improved significantly following recovery into the steady state. This observed improvement in GFR may be a reflection of the resolution of the patho-physiological factors operating during vasooclusive and hyperhemolytic crises.
In summary, the current study has clearly shown that the GFR reduction that occurs in SCD patients in vasooclusive and hyperhemolytic crises is reversible, following recovery into the steady state.
It is therefore advised that concerted efforts be put in place to prevent sickle cell crises and to aid speedy recovery from crisis’s state, in order to avoid the cumulative long-term effect of incessant crises on the renal function.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Survey of the haemoglobin genotypes in childhood at Ilorin. Niger J Paediatr. 1990;17:23-6.
- [Google Scholar]
- The presentation, management and prevention of crisis in sickle cell disease in Africa. Blood Rev. 1989;3:18-28.
- [Google Scholar]
- Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639-44.
- [Google Scholar]
- Glomerular filtration rate in Nigerian children with homozygous sickle cell disease. Niger J Med. 2002;11:23-5.
- [Google Scholar]
- Glomerular function in sickle cell disease patients during crisis. Afr J Med Med Sci. 1994;23:153-60.
- [Google Scholar]
- The effect of vasooclusive crisis on the glomerular filtration rate of children with sickle cell anaemia. Dissertation for fellowship of the West African College of Physicians thesis
- [Google Scholar]
- The sickle cell diseases. Clinical manifestations including the “sickle crisis”. Arch Intern Med. 1974;133:611-9.
- [Google Scholar]
- Hepatic function tests in children with sickle cell anaemia during vasoocclusive crisis. Cent Afr J Med. 1994;40:342-5.
- [Google Scholar]
- Pattern of infections among patients with sickle cell anaemia requiring hospital admission. Niger J Paediatr. 1983;10:13-7.
- [Google Scholar]
- Sickle cell disease and other disorders of abnormal haemoglobin. In: Miller DR, Baech RL, eds. Blood Diseases of Infancy and Childhood. Philadelphia USA: CV Mosby Co; 1990. p. :387-427.
- [Google Scholar]
- Measurement of renal function during growth in infancy and childhood. In: McCrory WM, ed. Developmental Nephrology. Havard: Cambridge Press; 1972. p. :95-108.
- [Google Scholar]
- The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-90.
- [Google Scholar]
- Renal function. In: Burtis CA, Ashwood ER, eds. Tietz Textbook of Clinical Chemistry. Philadelphia: WB Saunders Company; 1999. p. :1241-6.
- [Google Scholar]
- Phorbol ester stimulation increases sickle erythrocyte adherence to endothelium: A novel pathway involving alpha 4 beta 1 integrin receptors on sickle reticulocytes and fibronectin. Blood. 1996;88:4348-58.
- [Google Scholar]
- Sickle erythrocyte-endothelial interactions in microcirculation: The role of von Willebrand factor and implications for vasoocclusion. Blood. 1993;81:2429-38.
- [Google Scholar]
- Platelet function and survival in sickle cell disease. J Lab Clin Med. 1973;82:44-53.
- [Google Scholar]
- Elevated plasma levels of fibrinopeptide A during sickle cell anemia pain crisis - Evidence for intravascular coagulation. Am J Hematol. 1978;5:183-90.
- [Google Scholar]
- Nephropathy associated with sickle cell anemia: An autologous immune complex nephritis. I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am J Med. 1975;58:382-7.
- [Google Scholar]
- Nephropathy associated with sickle cell anemia: An autologous immune complex nephritis. II. Clinicopathologic study of seven patients. Am J Med. 1975;59:650-9.
- [Google Scholar]
- Norepinephrine decreases planar surface of rat mesngial cell. Kidney Int. 1987;31:424.
- [Google Scholar]
- Evidence for glomerular action of ADH and dibutyryl cyclic AMP in rat. Am J Physiol. 1977;233:102-7.
- [Google Scholar]
- Morbidity of homozygous sickle cell anaemia in Nigerian children. J Trop Pediatr. 1983;29:104-11.
- [Google Scholar]







