Translate this page into:
Influence of steroid maintenance on the outcomes in deceased donor kidney transplant recipients experiencing delayed graft function
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Delayed graft function (DGF) is a risk factor for poor long-term graft and patient survival after kidney transplantation. The aim of our study was to explore the beneficial effect of steroid maintenance on outcomes in deceased donor kidney (DDK) transplant recipients with DGF. Using organ procurement and transplant network/United network of organ sharing (OPTN/UNOS) database, we identified adult patients who developed DGF following DDK transplantation performed between January 2000 and December 2008. They received induction with rabbit antithymocyte globulin (r-ATG), alemtuzumab or an interluekin-2 receptor blocker (IL-2B) and were discharged on a calcineurin inhibitor (CNI)/mycophenolate (MMF) based immunosuppression with or without steroids. Adjusted graft and patient survivals were compared between steroid versus no steroid groups for each induction modality. Median follow-up was 29.6 months for the 10,058 patients who developed DGF. There were 5624 patients in r-ATG (steroid, n = 4569, no steroid, n = 1055), 819 in alemtuzumab (steroid, n = 301, no steroid, n = 518) and 3615 in IL-2B (steroid, n = 3380, no steroid, n = 235) groups. Adjusted graft survivals were similar for steroid versus no-steroid groups in patients who received r-ATG (HR: 0.98, 95% CI 0.85-1.13, P = 0.75), alemtuzumab (HR 0.88, 95% CI 0.65-1.19, P = 0.41), and IL-2B (HR 1.01, 95%CI 0.78-1.30, P = 0.96) inductions. The adjusted patient survivals were also similar in r-ATG (HR: 1.19, 95% CI 0.96-1.46, P = 0.19), alemtuzumab (HR: 0.89, 95% CI: 0.57-1.39, P = 0.96), and IL-2R (HR: 1.07, 95% CI: 0.77-1.49, P = 0.96) groups. Our study failed to show any significant graft or patient survival benefits associated with steroid addition to CNI/MMF regimen in DDK recipients with DGF. This may be related to the early immunogenic and non-immunogenic allograft damage from DGF with long-term consequences that are unaltered by steroids.
Keywords
Deceased donor transplantation
delayed graft function
graft survival
steroid maintenance
Introduction
Delayed graft function (DGF) is associated with poor long-term graft and patient outcomes following deceased donor kidney (DDK) transplantation. Based on a meta-analysis of 34 studies, patients with DGF had a 41% increased risk of graft loss, 38% relative increase in the risk of acute rejection (AR) and a 1.53 times risk for death with a functioning graft when compared to patients without DGF.[12] Despite a strong association between DGF and poor graft and patient survival, very few interventions exist to minimize this injury, such as reducing the cold and warm-ischemia times, keeping the patients euvolemic and avoidance of nephrotoxic insults. Improvements in donor and recipient management as well as diagnostic and therapeutic modalities seem neither to have reduced the overall incidence of DGF nor mitigated its short-term and long-term effects. This might partly be explained by the expansion of wait-list, older recipients, heightened interest in the use of expanded-criteria donor (ECD) as well as donation after cardiac death (DCD) kidneys and organs with extended cold ischemia times (CIT).[34]
DGF is predominantly the result of ischemic injury to the graft in the perioperative period, which is further aggravated by reperfusion injury, a multifactorial event in which immunologic factors play a crucial role. Ischemia-reperfusion injury can cause increased cytokine production and major histocompatibility complex class I and II expression on antigen presenting cells leading to increased allograft immunogenicity. This process can contribute to the development of AR, chronic allograft nephropathy and tubular-epithelial injury causing accelerated interstitial fibrosis and tubular atrophy.[567] It is intuitive to think that enhanced immunosuppression might be potentially beneficial in kidney transplant recipients experiencing DGF by halting or minimizing the immune activation associated with it. We aimed to explore whether the addition of corticosteroids to a calcineurin inhibitor (CNI)/mycophenolate mofetil (MMF) based maintenance immunosuppression in kidney transplant recipients would mitigate the increased allograft immunogenicity associated with DGF with resulting favorable graft and patient outcomes.
Materials and Methods
The study protocol was approved by the Institutional Review Board and was performed in accordance with the ethical standards laid down by the Declaration of Helsinki as well as Declaration of Istanbul. Using organ procurement and transplant network/United network of organ sharing (OPTN/UNOS) database, we identified patients older than 18 years who developed DGF following DDK transplants performed between January 1, 2000, and December 31, 2008. DGF was defined as the need for dialysis within 1st week of transplantation. Patients received induction therapy with rabbit antithymocyte globulin (r-ATG), alemtuzumab, or an interluekin-2 receptor blocking agent (IL-2B, either basiliximab or daclizumab) and were discharged on a CNI/MMF-based maintenance immunosuppression regimen with or without steroids. Under each induction category, patients were divided into two groups: Those who underwent early steroid withdrawal and those who were continued on maintenance steroid. Patients were included in the early steroid withdrawal group if they were discharged from the initial transplant admission without steroid. Patients were excluded from the analysis if they received multiorgan transplants, more than one, a different or no induction agents.
Demographic variables for the different induction groups were collected. Graft was considered failed when one of the following occurred: Need for maintenance dialysis, re-transplantation or patient death. Because demographic characteristics in the induction groups varied substantially, we decided to use an adjusted model in the analysis. Adjusted graft and patient survivals were compared between the steroids versus no steroid groups for each induction modality. Multivariate analysis using a Cox regression model was utilized to evaluate the independent influence of steroids on graft and patient outcomes. Confounding variables included in the analysis were: Donor related: Age, gender, ECD kidney, DCD kidney, death from cerebrovascular accident recipient related: Age, African American race, diabetes mellitus, dialysis duration, percentage of peak panel reactive antibody (PRA) titer, human leukocyte antigen mismatch, tacrolimus versus cyclosporine as the maintenance CNI agent, Transplant related: CIT, AR in first 12 months, previous transplant, transplant year.
Statistical analysis
Comparisons among groups were made using two-sided t-test for continuous variables and Chi-square test for categorical variables. When there were missing data for different variables/risk factors, we assumed absence of the risk factor for the purpose of analysis. Less than 2% of the data were missing for different variables (except for treated AR where 20-25% of data were missing) used in the analysis. Adjusted over all graft and patient survivals were calculated and compared for steroid versus no steroid groups for each induction modality after correcting for the confounding variables (listed above). Multivariate analysis was used with calculation of hazard ratio (HR) and 95% confidence intervals (CI) to evaluate the relative risks of various confounding variables in predicting both graft and patient outcomes. A P value of less than 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS software version 14.
Results
Median follow-up was 29.6 months (range: 10.7-60.1 months). There were 42,851 patients who received DDK transplant between January 2000 and December 2008 by using induction with r-ATG, alemtuzumab or an IL-2B agent. Among these patients, 10,058 developed DGF during the study period. Out of the 10,058 patients, 5624 received r-ATG (maintenance steroid = 4569, no steroid = 1055), 819 received alemtuzumab (maintenance steroid = 301, no steroid = 518) and 3615 an IL-2B agent (maintenance steroid = 3380, no steroid = 235) for induction. Demographic characteristics of the recipients with DGF by the induction type and stratified by steroid maintenance are shown in Table 1.
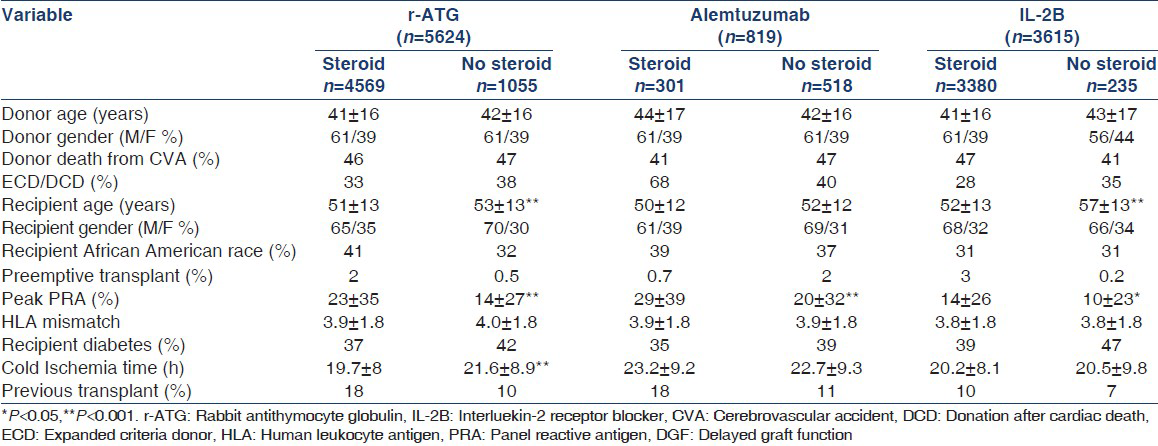
In the r-ATG induced patients, recipient age and CIT were higher in no steroid group compared to maintenance steroid group (P < 0.001). In IL-2B induced patients, the recipient age was higher in no steroid group compared to maintenance steroid group (P < 0.001). For all three induction modalities, peak PRA titer was significantly lower in no steroid group compared to maintenance steroids (P < 0.05).
Impact of maintenance steroid immunosuppression on graft survival
Adjusted graft survival for maintenance steroid versus no steroid groups were similar in r-ATG (HR: 0.98, 95% CI 0.85-1.13, P = 0.75), alemtuzumab (HR 0.88, 95% CI 0.65-1.19, P = 0.41), and IL-2R (HR 1.01, 95%CI 0.78-1.30, P = 0.96) induced patients [Figure 1].

- Adjusted graft survival by induction type: Rabbit antithymocyte globulin (a), alemtuzumab (b), interluekin-2 receptor blocker (c)
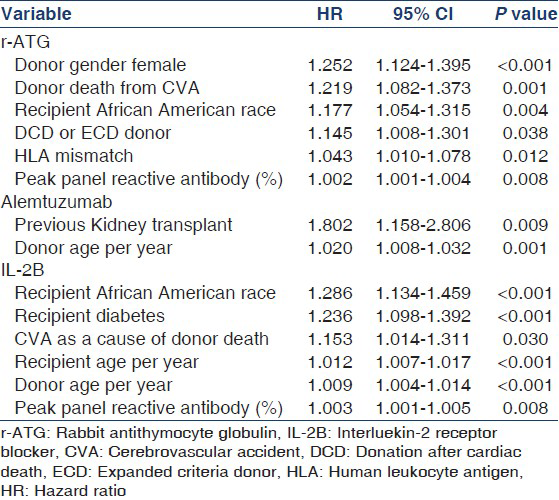
The strongest predictors for graft loss in the multivariate model for each induction modality are shown in Table 2.
Impact of maintenance steroid immunosuppression on patient survival
The adjusted patient survival in r-ATG (HR: 1.19, 95% CI 0.96-1.46, P = 0.19), alemtuzumab (HR: 0.89, 95% CI: 0.57-1.39, P = 0.96), and IL-2R (HR: 1.07, 95% CI: 0.77-1.49, P = 0.96) were similar in the maintenance steroids versus no steroid groups [Figure 2].
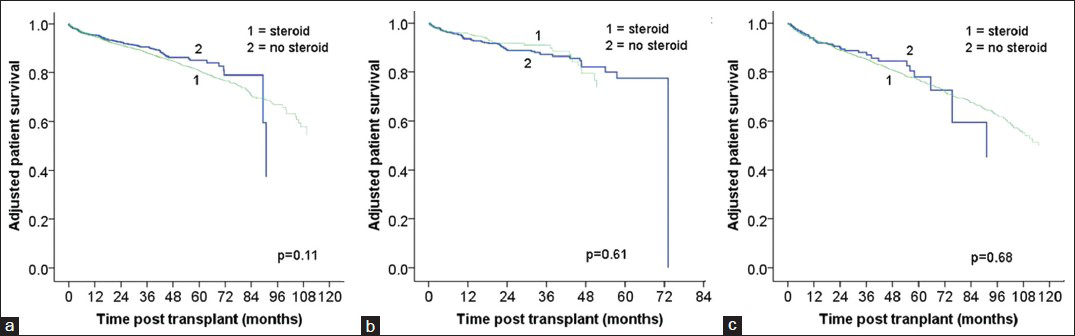
- Adjusted patient survival by induction type: Rabbit antithymocyte globulin (a), alemtuzumab (b), interluekin-2 receptor blocker (c)
The strongest predictors for patient death in the multivariate model for each induction modality are shown in Table 3.

Acute rejection rates
The rates of AR at 12 months post-transplant were significantly higher in steroid maintenance group compared to no steroids for alemtuzumab (13% versus 8%, P = 0.018) and IL-2R (15% versus 9%, P = 0.009) induction modality. However, with r-ATG induction there was no significant difference in the rates of AR in maintenance steroid group compared to no steroids. (10% versus 9.6%, P = 0.43).
Discussion
There is a paucity of data on the influence of maintenance steroids on outcomes in kidney transplant recipients who experience DGF and these patients are generally maintained on steroids with the hope of improving outcomes. Our study found no significant graft or patient survival benefits associated with the addition of maintenance steroids to a CNI/MMF based immunosuppressive regimen in DDK transplant recipients who developed DGF regardless of the induction type they received.
Current maintenance immunosuppression protocols following kidney transplantation typically include a CNI and MMF based regimen with or without steroids. Registry reports and meta-analyses have noted increased AR rates after rapid steroid withdrawal compared to steroid maintenance with no measurable influence on outcomes in kidney transplant recipients.[891011] Although, long-term glucocorticoids are currently administered to the majority of kidney transplant recipients, strategies aimed at minimizing or avoiding these agents are increasingly used. For instance, approximately 30% of patients were discharged on steroid free maintenance immunosuppressive regimen in 2009.[12]
A 5-year prospective randomized controlled trial by Woodle et al., reported similar long-term renal allograft survival and function in early steroid withdrawal group (within 7 days of transplantation) compared to steroid maintenance group. Incidence of mild biopsy confirmed AR was higher in steroid withdrawal group. Early steroid withdrawal provided an improvement in cardiovascular (CV) risk profile. However, this study excluded patients who experienced DGF.[13] The Meta-analysis of 34 studies by Knight et al., showed a small increase in the rate of AR in steroid avoidance/withdrawal group, with no measurable effect on graft or patient survival, and this group had significant benefits in terms of CV risk factors. This analysis did not have any information on the incidence or outcomes in DGF.[14] A large single center retrospective analysis involving 1241 primary kidney transplant recipients by Rizzari et al., showed similar patient, graft, death-censored graft, and AR free survival rates at 10 years for rapid steroid discontinuation group compared to historic controls on maintenance steroids. This study also noted a significant reduction in the incidence of new onset diabetes after transplantation (NODAT), cataracts and avascular necrosis in rapid steroid discontinuation group. However, this study compared prednisone related side effects to historic control group maintained on relatively higher doses of prednisone (0.1-0.15 mg/kg). DGF developed in 2.5% of living and 17% of DDK transplant recipients. Patients who developed DGF received extended course of r-ATG (up to a maximum of 10 doses) along with a small dose of prednisone (5 mg/day). Prednisone was discontinued with the last does of r-ATG. A subset analysis of outcomes in these patients was not reported.[15] Khwaja et al., studied rapid steroid discontinuation in a cohort of 79 kidney transplant recipients at increased immunological risk (patients with second/third transplant, DGF, peak PRA of >10%, African American race) and reported similar graft, patient and AR free survival rates when compared to conventional steroid based immunosuppression regimens at 3 years post-transplant. This study had only 26 patients with DGF thus precluding any meaningful conclusions.[16] A multicenter prospective trial by Vincenti et al., involving 336 de novo kidney transplant recipients randomized patients to steroid free, rapid steroid withdrawal (by post-operative day 7), or standard steroid therapy (5-10 mg/d of prednisone) for maintenance immunosuppression in addition to cyclosporine A and MMF after basiliximab induction. This study had about 22% of patients with DGF in each arm. A subset analysis of this group showed no significant difference in the composite endpoint of AR rates, graft and patient survivals, with steroid minimization compared to steroid maintenance. However, this study was not adequately powered to detect significant differences in DGF subgroup.[17] A retrospective analysis of the scientific registry of transplant recipients looking at the outcomes of de novo steroid free immunosuppression in 16491 adult kidney transplant recipients from 2000 to 2008 by Luan et al., showed reduced risk of graft failure and death at 1 year and 4 years in steroid free group. Outcomes related to subgroup of patients with DGF were not reported in this analysis.[18]
Current data suggests that in patients who are at low immunological risk and receive induction therapy, corticosteroids could be discontinued during the 1st week after transplantation.[19] Whether a steroid sparing strategy can be used safely in kidney transplant recipients who develop DGF is unknown. Most studies carried out until now looking at steroid withdrawal protocols either eliminated patients with DGF, considering them as high immunological risk or had very few patients with DGF and were not adequately powered to validate the significance of results in the subgroup analysis.
To our knowledge, the present study is the first analysis looking specifically on the impact of steroid maintenance on the outcomes of DDK transplant recipients who experienced DGF utilizing a large nationally representative data base. We found no clinically demonstrable beneficial effects for steroid maintenance in these patients in terms of graft and patient survivals as well as AR. It is well established that, ischemia-reperfusion injury around the time of transplant plays a crucial role in the development of DGF causing increased allograft immunogenicity. Steroid use in such patients seems reasonable to counter the enhanced immunogenicity. The results of our study did not support this hypothesis. Factors such as prolonged cold-ischemia time, oxidative stress, donor age, and dialysis vintage, can increase the risk for DGF by incurring non-immunological damage.[20] It is possible that DGF could be resulting in significant immunological and non-immunological damage of the renal allograft that is not altered by steroid use to any clinically meaningful level. Moreover, long-term steroid use may contribute to additional CV morbidity, including NODAT, worsening of established diabetes, osteopenia, avascular necrosis, hypertension, dyslipidemia, and possibly increasing the risk of dying with a functioning graft. Interestingly our analysis also noted increased rates of AR at 1 year in steroid maintenance group for alemtuzumab and IL-2R. This could represent a selection bias since steroid group had a significantly higher PRA% at the time of transplant and may have been preferentially placed on steroid maintenance because of a high risk for AR. We did not see this difference in the r-ATG group.
Large number of patients involving multiple transplant centers nationally and a fairly long follow-up increases the validity of our results. Our study has several limitations. It is a retrospective analysis and can demonstrate only association but not causation. Some early steroid withdrawal protocols withdraw steroids at 7 days post-transplant. If these patients were discharged in less than 7 days post-transplant, they would be falsely categorized as being on steroids. Residual confounding factors can still exist despite using an adjusted model, and longitudinal changes in maintenance immunosuppression likely existed that are not captured. We did not have data on the late AR rates, which can adversely impact graft outcomes.[21] Possibility of a type 2 error cannot be excluded.
In summary, our analysis did not support the preferential use of steroid maintenance in DDK transplant recipients experiencing DGF following induction therapy and CNI/MMF maintenance. A prospective randomized controlled trial of sufficient size and follow-up, which specifically looks at patients who developed DGF, is needed to evaluate the role of steroid maintenance on their outcomes.
Acknowledgment
Presented in part as a poster at the National Kidney Foundation Spring Clinical meeting, May 2012, Washington, DC.
Source of Support: This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government
Conflict of Interest: None declared.
References
- Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039-47.
- [Google Scholar]
- Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153-61.
- [Google Scholar]
- Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-74.
- [Google Scholar]
- Tackling the shortage of donor kidneys: How to use the best that we have. Am J Nephrol. 2003;23:245-59.
- [Google Scholar]
- Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation. 2002;74:916-23.
- [Google Scholar]
- Why do we reject a graft? Role of indirect allorecognition in graft rejection. Kidney Int. 1999;56:1967-79.
- [Google Scholar]
- Steroid-free maintenance immunosuppression in kidney transplantation: Is it time to consider it as a standard therapy? Kidney Int. 2009;76:825-30.
- [Google Scholar]
- Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009;1:CD005632. doi: 10.1002/14651858.CD00563.2pub2
- [Google Scholar]
- Renal transplantation with early steroid withdrawal. Pediatr Nephrol. 2009;24:243-51.
- [Google Scholar]
- Available from: http://www.ustransplant.org
- A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564-77.
- [Google Scholar]
- Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1-14.
- [Google Scholar]
- Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol. 2012;7:494-503.
- [Google Scholar]
- Rapid discontinuation of prednisone in higher-risk kidney transplant recipients. Transplantation. 2004;78:1397-9.
- [Google Scholar]
- A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8:307-16.
- [Google Scholar]
- Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression. Am J Transplant. 2009;9:160-8.
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
- [Google Scholar]
- Delayed graft function and long-term outcome in kidney transplantation. Transplant Proc. 2012;44:1879-83.
- [Google Scholar]
- The implications of acute rejection for allograft survival in contemporary U. S. kidney transplantation. Transplantation. 2012;94:369-76.
- [Google Scholar]







