Translate this page into:
Cyclosporine/ketoconazole reduces treatment costs for nephrotic syndrome
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cyclosporine A (CyA) is an effective agent for the treatment of glucocorticoid-dependent idiopathic nephrotic syndrome (GCDNS), but costs are prohibitive in resource-poor societies. The objectives of this study were to evaluate the efficacy and safety of reducing the dose of CyA by co-administering ketoconazole. A prospective study targeting children 2-18 years of age with GCDNS in remission with CyA monotherapy was conducted. CyA dose was reduced by 50% and ketoconazole was added at 25% of the recommended therapeutic dose, and the drug levels and therapeutic and adverse effects (AE) were monitored. Continued combined therapy after completion of the 4-week trial period was offered. Ten patients (median age 9.5 years, range 3.0-16.0 years) were enrolled in the study. At week 4, the CyA dose was 2.2 ± 0.7 mg/kg/day compared with 5.6 ± 0.9 mg/kg/day at enrolment (P < 0.0001). No AE were noted. All patients continued ketoconazole treatment for at least 3 months. CyA drug cost savings were 61%, and approximately 60% with ketoconazole cost included. The combination of an expensive immunosuppressive drug with a cheap metabolic inhibitor reduced the treatment costs by> 50% without increased adverse events or drug monitoring needs. This intervention demonstrates how access of patients with limited resources to needed drugs can be improved by interference with physiological drug elimination.
Keywords
Azoles
co-administration
cyclosporine
India
nephrotic syndrome
pediatric
Introduction
Idiopathic nephrotic syndrome (INS) is the most common chronic kidney disease in childhood worldwide. The majority of children with INS have minimal renal lesions by histology (minimal change disease) and respond to treatment with high-dose prednisone. Thirty percent to 50% of children with INS will have frequent relapses of proteinuria and/or become glucocorticoid dependent, while a minority shows primary glucocorticoid resistance, including patients with a histological diagnosis of focal segmental glomerulosclerosis.[123] Cyclosporine A (CyA) is an effective second-line agent for the treatment of glucocorticoid-dependent and resistant forms of INS.[24] However, costs of this immunosuppressant are prohibitive in resource-poor countries.[56]
There is a need to explore options that may decrease the costs of expensive immunosuppressive medications while maintaining safety and efficacy of therapy. Ketoconazole, an antifungal agent, increases CyA blood levels through the inhibition of cytochrome P450 microsomal enzymes.[789] Some transplant centers reported the intentional use of drugs that interfere with the P450 enzyme system, such as diltiazem, erythromycin or the azole ketoconazole, to reduce the CyA dose while maintaining therapeutic blood cyclosporine concentrations.[6810111213] Azoles inhibit fungal growth by competing with and blocking the cytochrome P450, family 3, subfamily A (cytochrome P3A4)-dependent enzyme lanosterol 14-α-demethyase, which catalyzes the synthesis of the major sterol component of fungal plasma membrane, ergosterol. The nonselective azole, ketoconazole, cross-inhibits mammalian CYP3A4 resulting in up to 80% reduction of the metabolism of CyA.[14]
Pharmacokinetic studies in solid organ transplant recipients using a combination of ketoconazole and CyA demonstrated a three-fold increase of the area under the curve of CyA that allowed reductions of the CyA dose by as much as 70%.[15] The choice of CyA-sparing agents will be influenced by the safety of the drug in combination with CyA, the frequency of adverse effects (AE) and potential additional therapeutic benefits by the second agent.[161718] Children and adults display different pharmacokinetics of CyA and their metabolic inhibitors.[19] Several studies described the co-administration of CyA and ketoconazole in pediatric solid organ transplant recipients.[1011131520] While the concept of combining CyA and ketoconazole is not new, its benefit and safety in children with nephrotic syndrome in developing countries has not been adequately studied. The only larger dataset originates from a single center.[202122] We therefore initiated a pilot study to assess the feasibility and safety of this combination therapy in a pediatric cohort in Southern India.
The objectives of the study were to evaluate the efficacy and safety of co-administered ketoconazole to reduce the therapeutic dose of CyA in children with glucocorticoid-dependent nephrotic syndrome and to estimate the resulting cost savings of CyA therapy in this population. The secondary aims of the study were to assess the acceptance and feasibility of this approach in our population.
Materials and Methods
Study design
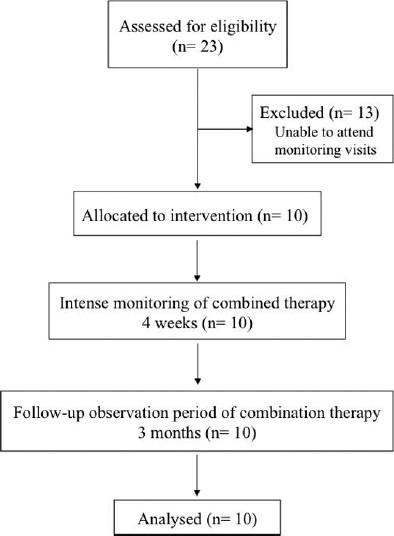
This is a prospective interventional study conducted in the Division of Pediatric Nephrology at St John's Medical College Hospital, Bangalore, between June 2009 and June 2010. Ethical committee clearance was obtained from the institutional ethical review board. All parents provided informed consent prior to enrollment.
Inclusion and exclusion criteria
Children between 2 and 18 years of age with glucocorticoid (steroid)-dependent INS were eligible. Glucocorticoid dependence was defined as the occurrence of two consecutive relapses of nephrotic-range proteinuria during or within 2 weeks after discontinuing alternate-day prednisone therapy.[23] Children whose parents found it difficult to bear the cost of CyA therapy were included (purposive sampling). Recruitment was limited to patients who were in remission and off glucocorticoids for at least 4 weeks and who had been treated for at least 3 months with CyA monotherapy with stable trough levels <150 ng/mL. Children with infections, renal insufficiency (creatinine > 90 μmol/L), systemic hypertension (blood pressures > 95th centile for age and height) and leukocytopenia (total white blood cell count < 4 × 109/L) were excluded.
Study protocol
The study period comprised 4 weeks of intense monitoring, followed by 3 months of continued observation [Figure 1]. Parents were given the option to continue the combined therapy after completion of the trial. The standard initial CyA dose was 5-6 mg/kg/day orally in two divided doses (Sandimmune Neoral, Novartis). It was modified to achieve trough levels between 100 and 150 ng/mL. When ketoconazole was added, the CyA dose was reduced by 50% of the actual dose. We prescribed ketoconazole (Nizoral, Janssen Cilag) at ~25% of the recommended, weight-adjusted dose for its antifungal (systemic) use (about 1.5 mg/kg/day). The decision was based on our pre-trial observation with the full therapeutic ketoconazole dose, which resulted in excessive CyA trough levels of > 300 ng/mL (n = 3). Previous investigators used a daily dose of 50 mg ketoconazole without specifying age or body weight of their pediatric probands.[2122] Ketoconazole was dispensed as tablets or powdered sachets prepared by our pharmacy, and CyA was given as tablets or suspension, as appropriate.
- Study protocol
Clinical and laboratory monitoring
A standardized questionnaire was used to record and evaluate patients’ adherence to therapy and occurrence of AEs of either drug, including gastrointestinal disturbances, headache, hypertension, seizure and infection. Weekly laboratory tests included urine albumin, CyA trough level, serum creatinine, total leukocyte counts and liver enzymes for four consecutive weeks.
Drug monitoring and dose adjustment
CyA trough levels were analyzed in whole blood using an enzyme-multiplied immunoassay technique (EMIT) assay kit, Syva EMIT (Syva Co., San Jose, California). This assay shows insignificant cross-reactivity for CyA metabolites AM1, AM19 and AM4N, but some reactivity with metabolite AM9.[2425] If the CyA trough level (C0; sample taken ½ h prior to the morning dose) was > 150 ng/mL, the CyA dose was to be reduced further by 25% of the actual dose. When the trough level was <100 ng/mL, the ketoconazole dose was to be increased by 0.5 mg/kg/day.
Study termination
Children were observed for a minimum of 3 months following the initial study period. At the end of the 3-month follow-up, we performed a clinical assessment, measured urine albumin excretion and analyzed the costs of CyA therapy. Probands exited the study if they reached one or more of the following critical parameters: Relapse of proteinuria (dipstick urine albumin 2+ or greater for three consecutive days), rise of liver transaminases to more than double the upper reference range, leukocytopenia, renal dysfunction (doubling of serum creatinine) or CyA trough level > 200 ng/mL.
Economic assessment
Total costs were calculated per month of treatment. The original CyA dose was compared with the dose at the end of the first 4-week period, as were the costs for the added ketoconazole. Drug level monitoring was part of the study protocol and costs were not included into the calculation.
Statistical analysis
Continuous variables were expressed as mean and standard deviation or as median and range when a normal distribution could not be assumed. Categorical variables were expressed as percentages. Pearson's correlation coefficient was used to find the correlation between C0 and creatinine. Student's t test was used for CyA dose comparison. Analyses were performed using SPSS (version 12).
Results
Of 23 eligible children, consent was obtained from 10 patients (six girls). Thirteen children were not recruited because they were unable to attend the required monitoring visits at the hospital, usually for economic reasons [Figure 1]. The median age of the study cohort was 9.5 years (range 3.0-14.3 years). All 10 patients remained in remission during the study and subsequent observation periods. The mean CyA trough level immediately prior to enrollment was 101.6 ± 25.19 ng/mL. The mean trough level nominally peaked at 149.8 ± 58.2 ng/mL, 1 week after adding ketoconazole and decreasing the CYA dose, and then stabilized over the study period [Figure 2].

- Cyclosporine trough levels
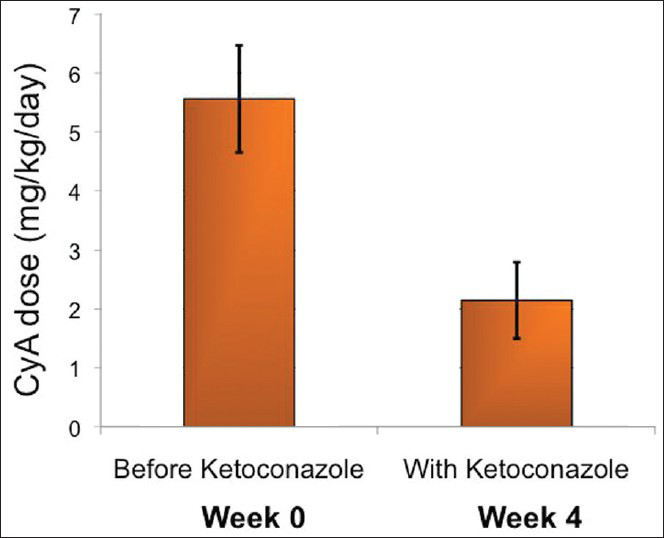
The mean CyA dose to maintain remission prior to adding ketoconazole (at the time of recruitment) was 5.56 ± 0.91 mg/kg/day compared with a dose of 2.15 ± 0.65 mg/kg/day at 4 weeks of combined therapy [P < 0.0001; Figure 3]. The average ketoconazole dose in the 1st week was 1.74 ± 0.8 mg/kg/day. All patients opted to continue the combination therapy for at least 3 months. No further CyA and ketoconazole dose changes were made during the 3 months of follow-up. Remission was maintained in all children throughout the 4-week study and 3-month extension period despite a CyA dose reduction by 61%, demonstrating the efficacy of the combination therapy.
- Reduction in dosage of cyclosporine after adding ketoconazole
Safety of the CyA/ketoconazole co-administration
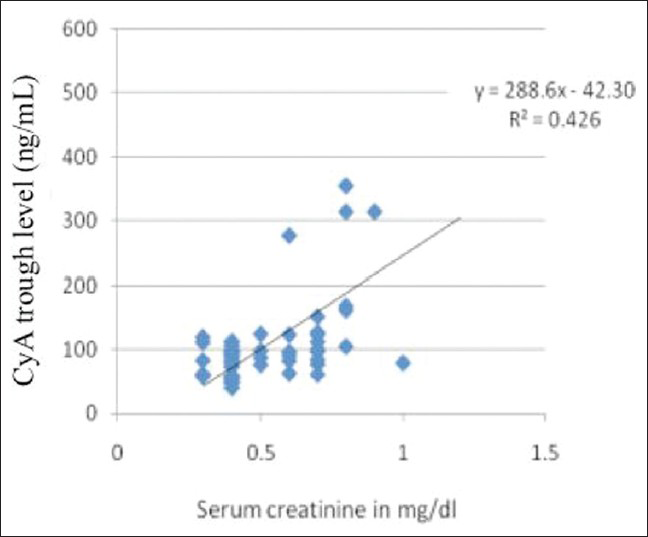
The mean creatinine concentrations at the time of recruitment and at the end of the 4-week study period were 35 ± 9 μmol/L (0.4 ± 0.1 mg/dL) and 44 ± 9 μmol/L (0.5 ± 0.1 mg/dL). The difference was not significant. We found a moderate correlation (r2= 0.42) between CyA trough (C0) and serum creatinine concentrations [Figure 4]. No AEs related to CyA or ketoconazole were observed in the patient cohort.
- Correlation between cyclosporine trough levels and serum creatinine
Cost savings
The addition of ketoconazole reduced the average calculated cost of CyA therapy by 61% based on the average reduction of the CyA dose [Figure 3]. Patients were not charged for the added ketoconazole during the study. The cost reduction for CyA would be off-set by approximately 1% after adjustment for the ketoconazole dose at the end of the 4-week study period.
Discussion
The idea of exploiting the interaction between ketoconazole and CyA is not new,[717] but has regained relevance for economic considerations.[62627] Costs constrain medical practice worldwide, but more dramatically in developing countries.[27] While many proposed strategies that aim at mitigating access disparities to needed drugs require structural changes, utilization of physiological aspects of drug metabolism can be implemented immediately, provided these strategies are safe and effective and are not offset by expensive clinical or laboratory monitoring. Combination of drugs with additional pharmacological properties, such as antimicrobial effects, has been cited as an additional advantage, e.g., if the target patient population is immunosuppressed due to the disease or its treatment.
Here, we report the first, although small, prospective study employing the CYP450 pathway inhibitor ketoconazole to reduce the costs for an important, expensive second-line agent in the treatment of children with glucocorticoid-dependent nephrotic syndrome. Treated children maintained remission of proteinuria and normal renal function throughout the study and observation period and experienced no CyA or ketoconazole-related AEs. Specifically, ketoconazole-associated vomiting, flatulence, diarrhea, gynecomastia and pruritus, or (transient) increases in hepatic aminotransferases, cholestasis or mixed hepatotoxicity[28] were not seen in our patient cohort treated with low-dose ketoconazole.
Previously reported studies in children with nephrotic syndrome are limited to a single center in Egypt.[2122] These authors reported clinical improvement, including the degree of proteinuria and renal function in the majority of children with glucocorticoid-resistant nephrotic syndrome treated with this medication combination. El-Husseini et al., prescribed a dose of 50 mg/day of ketoconazole in combination with CyA for children with INS and reported a favorable efficacy and safety profile.[21] We used a lower sub-therapeutic dose of ketoconazole (about 1.5 mg/kg/d, capped at 50 mg/day) to avoid excessive CyA levels secondary to overzealous metabolic pathway inhibition and ketoconazole dose-related toxicity. At the end of the study period of 4 weeks, although we had a few C0 trough levels of <100 ng/dL, we did not make any further changes in doses due to economic reasons for drug level monitoring although all patients maintained remission.
The mechanism of the interaction between CyA and ketoconazole is thought to be the avid nonselective binding of early azoles to the mammalian microsomal monooxygenase cytochrome P-450 enzyme system resulting in the inhibition of the metabolism of CyA.[1428] Other proposed mechanisms include altered absorption of CyA, competition for excretion, change in the volume of distribution of CyA and altered protein binding.[29] CyA undergoes extensive biotransformation to multiple metabolites. The primary AM1, AM4N and AM9 metabolites are formed by CYP3A4. AM9 formation is also catalyzed by the polymorphic CYP3A5, the only CYP3A isoform expressed in renal tissue. The latter is not blocked by ketoconazole and therefore should not lead to falsely high measurements with immunoassays.[14] For rationale therapeutic inhibition of the CyA metabolism, additional studies can be envisioned using liquid chromatography and mass spectrometry to accurately determine the blood concentrations of active CyA metabolites.
The cost of medications is a major factor governing treatment adherence in low-income countries. Adherence has not been the focus of our limited study. However, our results reinforce the concept that the exploitation of drug-related physiological principles can reduce drug costs and, by inference, improve patient adherence.
In conclusion, ketoconazole was effective in maintaining remission at reduced CyA drug dosing and cost. This therapeutic intervention has a place in treating children with nephrotic syndrome deserving effective second-line therapy in the developing world. Until further information becomes available, close clinical and basic laboratory monitoring is advised to meet the challenges of reducing therapeutic costs while providing effective and safe treatment.
Source of Support: The study was supported by a research grant provided by the St. John's research society, St. John's Medical College, Bangalore.
Conflict of Interest: None declared.
References
- New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol. 2011;26:881-92.
- [Google Scholar]
- Recurrent focal segmental glomerulosclerosis: A discrete clinical entity. Int J Nephrol 2012 2012 246128
- [Google Scholar]
- Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis. 2009;53:760-9.
- [Google Scholar]
- Cyclosporine A induced remission of relapsing nephrotic syndrome in children. Kidney Int. 1988;33:729-34.
- [Google Scholar]
- The use of a cyclosporin-ketoconazole combination: Making renal transplantation affordable in developing countries. Eur J Clin Pharmacol. 2004;60:143-8.
- [Google Scholar]
- Pediatric clinical use of the ketoconazole/cyclosporin interaction. Pediatr Nephrol. 1994;8:492-3.
- [Google Scholar]
- Coadministration of ketoconazole to cyclosporin-treated kidney transplant recipients: A prospective randomized study. Am J Nephrol. 1995;15:493-9.
- [Google Scholar]
- Coadministration of ketoconazole and cyclosporine for kidney transplant recipients: Long-term follow-up and study of metabolic consequences. Am J Kidney Dis. 2001;37:510-7.
- [Google Scholar]
- The clinical and economic potential of cyclosporin drug interactions. Pharmacoeconomics. 1999;15:317-37.
- [Google Scholar]
- Everolimus versus azathioprine in a cyclosporine and ketoconazole-based immunosuppressive therapy in kidney transplant: 3-year follow-up of an open-label, prospective, cohort, comparative clinical trial. Transplant Proc. 2010;42:270-2.
- [Google Scholar]
- In vitro metabolism of cyclosporine A by human kidney CYP3A5. Biochem Pharmacol. 2004;68:1889-902.
- [Google Scholar]
- Recent contributions to transplantation at the University of Cincinnati. Clin Transpl 1991:159-78.
- [Google Scholar]
- Cyclosporin and ketoconazole, drug interaction or therapeutic association? Int J Clin Pharmacol Ther Toxicol. 1992;30:555-70.
- [Google Scholar]
- Ketoconazole to reduce the need for cyclosporine after cardiac transplantation. N Engl J Med. 1995;7(333):628-33.
- [Google Scholar]
- The use of other drugs to allow a lower dosage of cyclosporin to be used. Therapeutic and pharmacoeconomic considerations. Clin Pharmacokinet. 1997;32:357-67.
- [Google Scholar]
- Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther. 1999;66:66-75.
- [Google Scholar]
- Single-center experience with cyclosporine for treatment of idiopathic minimal change nephrotic syndrome in children. Iran J Kidney Dis. 2009;3:127-35.
- [Google Scholar]
- Concomitant administration of cyclosporine and ketoconazole in idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2004;19:2266-71.
- [Google Scholar]
- Co-administration of cyclosporine and ketoconazole in idiopathic childhood nephrosis. Pediatr Nephrol. 2004;19:976-81.
- [Google Scholar]
- Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int. 2007;72:1429-47.
- [Google Scholar]
- Evaluation of EMIT Cyclosporine Assay for use with whole blood. Clin Chem. 1993;39:2235-41.
- [Google Scholar]
- Lack of specificity of cyclosporine immunoassays. Results of a College of American Pathologists Study. Arch Pathol Lab Med. 2003;127:19-22.
- [Google Scholar]
- Briseño-Renteria G Is renal replacement therapy for all possible in developing countries? Ethn Dis. 2006;16:S270-2.
- [Google Scholar]
- International drug price comparisons: Quality assessment. Rev Panam Salud Publica. 2011;29:46-51.
- [Google Scholar]
- Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26:1730-44.
- [Google Scholar]







