Translate this page into:
Accuracy of spot urine protein creatinine ratio in measuring proteinuria in chronic kidney disease stage 3 and 4
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We studied the accuracy of spot urine protein creatinine ratio (SpUr-PCR) to assess 24 h urine protein excretion (24 h-UP) in patients with chronic kidney disease (CKD). A total of 100 proteinuric CKD patients of stages 3 and 4 were studied. 24 h urine was collected to measure 24 h-UP and creatinine. A random day time urine sample was analyzed to measure the PCR. A formula to estimate 24 h creatinine excretion was derived from linear regression analysis and a correction factor was introduced to assess whether this improves the accuracy of the SpUr PCR in predicting 24 h-UP. Accuracy of the SpUr-PCR was assessed by Pearson's correlation, regression analysis, and Bland Altman analysis. Mean age was 51.85 ± 12 years and 81% of the patients were male. SpUr-PCR predicted 24 h-UP with good accuracy (r = 0.86 on a data transformed to a logarithmic scale, P < 0.001) and there was a good agreement between these two measures of proteinuria. However, SpUr-PCR was inaccurate in the subgroup with nephrotic range proteinuria (r = 0.35, P = 0.062), but when a correction factor for 24-h urine creatinine (24 h-UCr) was introduced, the accuracy of SpUr-PCR improved significantly in this group (r = 0.45, P = 0.013). Introduction of the correction factor improved the degree of agreement between these two measures in women, but not the correlation. Overall, SpUr-PCR accurately predicted 24 h-UP. Adding a correction factor for 24 h-UCr improved correlation in the subgroup of patients with the nephrotic range proteinuria and the degree of agreement in female patients, and hence may be used in expressing proteinuria measured by SpUr-PCR to improve its accuracy in them.
Keywords
Chronic kidney disease
creatinine excretion
proteinuria
urine protein creatinine ratio
Introduction
Proteinuria contributes to progression of chronic kidney disease (CKD) by several mechanisms and interventions to reduce proteinuria improve the outcome.[12] Hence, accurate assessment of proteinuria is an essential part of management of CKD. 24 h urine collection is the gold standard to measure proteinuria. However, in clinical practice 24 h urine collection is cumbersome and also errors in the collection are seen in 10-20% of samples,[3] making it an unreliable measure of proteinuria. To circumvent this problem, Ginsberg et al.,[4] proposed measurement of protein to creatinine ratio (PCR) in a spot urine (SpUr) sample to predict 24 h proteinuria. The basis of this test was the fortuitous finding that creatinine excretion is approximately 1 g/1.73 m2/day, and the test assumes that creatinine excretion is uniform at 1 g/day in all individuals, which is likely to be incorrect. Creatinine excretion is variable within individuals and largely depends on muscle mass, which is influenced by several factors such as age, gender, body size, and diet.[56] In CKD patients, creatinine generation may be affected by the reduced muscle mass due to under-nutrition in advanced CKD.[5] Creatinine excretion in healthy as well as CKD populations of Asian Indians is unexplored and hence spot urine protein creatinine ratio (SpUr-PCR) to measure proteinuria remains invalidated in them.
Several studies have validated the accuracy of SpUr-PCR to reliably measure proteinuria; however, most of them were carried out in patients with normal or near normal glomerular filtration rate (GFR) and were reviewed recently.[7] Though most studies showed good accuracy of SpUr-PCR,[7] some showed poor agreement.[8] Few studies were performed to validate this test in advanced CKD and the number of patients studied was small.[4910111213]
We studied the accuracy of SpUr-PCR in predicting proteinuria in Asian Indian patient with CKD stages 3 and 4. We also studied the creatinine excretion in them to determine whether the addition of a correction factor for creatinine excretion would enhance the accuracy of this test.
Subjects and Methods
We studied 100 adult patients with the CKD stage 3 (N = 43) and 4 (N = 57) attending outpatient clinic, who consented for the study. Approval from the hospital ethics committee was obtained to conduct the study. Stable adult CKD patients with an estimated GFR between 15 and 60 ml/min/1.73 m2, assessed by 6-variable modification of diet in renal disease equation[14] and dipstick positive proteinuria were included in the study. We excluded patients with febrile illness, acute renal failure, urinary infection, gross hematuria, and pregnancy.
The correct method to collect 24 h urine was explained in detail to the patients. 24 h urine protein (24 h-UP) and 24 h urine creatinine (24 h-UCr) concentrations were measured in a urine sample thus collected. A day time random sample either soon before or after the collection of 24 h urine sample was collected and protein and creatinine concentrations were measured in it and SpUr-PCR was calculated by dividing urinary protein by urinary creatinine, both expressed as mg/dl. We preferred a day time sample to morning first void urine sample for the reason that it would be a better representative of proteinuria[4] and for the practical reason that it would facilitate rapid analysis in the laboratory. Urinary protein concentration was determined by colorimetric method with pyrogallol red and creatinine concentrations in serum and urine were determined by modified Jaffe method, in an automated ADVIA apparatus. Demographic data such as age, gender, cause of CKD, the presence of diabetes, and hypertension were collected. Measurement of blood urea, serum creatinine, serum albumin, and hemoglobin were carried out. Anthropometric measures such as weight, height, body mass index (BMI), triceps skin fold thickness (TSFT), scapular skin fold thickness, and mid arm circumference (MAC) were carried out. The body surface area was calculated using the Mosteller equation.[15]
Calculations
-
Estimation of creatinine excretion (mg/day) by Cockcroft and Gault (CG) formula:[16] (140-Age) × Weight in kg/5 in men and multiplied by 0.85 in women
-
Estimation of creatinine excretion (mg/day) by formula proposed by Ix et al.:[17] 879.89+ (12.51 × weight in kg) − (6.19 × Age) − (379.42 if female)
-
Mid arm muscle circumference (MAMC) was calculated by:[18] MAMC (cm) = (MAC in cm) − (π × [TSFT in cm/10])
-
Mid arm muscle area (MAMA) was calculated by:[18] MAMA (cm2) = ([MAMC]2/4π) − 10 for men; ([MAMC]2/4 π) − 6.5 for women.
Statistical analyses
Student t-test for quantitative variables and χ2 test for qualitative variables were used for comparison. The accuracy of SpUr-PCR in predicting 24 h-UP was assessed by (1) Correlation, which was tested using the Pearson correlation coefficient (r), (2) precision by r2 statistics (goodness of fit analysis) and, (3) Degree of agreement by Bland Altman analysis. Linear regression analysis was performed to identify variables affecting 24 h-UCr. P <0.05 was selected as the level of significance. Statistical analysis was carried out using the Statistical Package for Social Science version 17.1 (Chicago, IL, USA).
Results
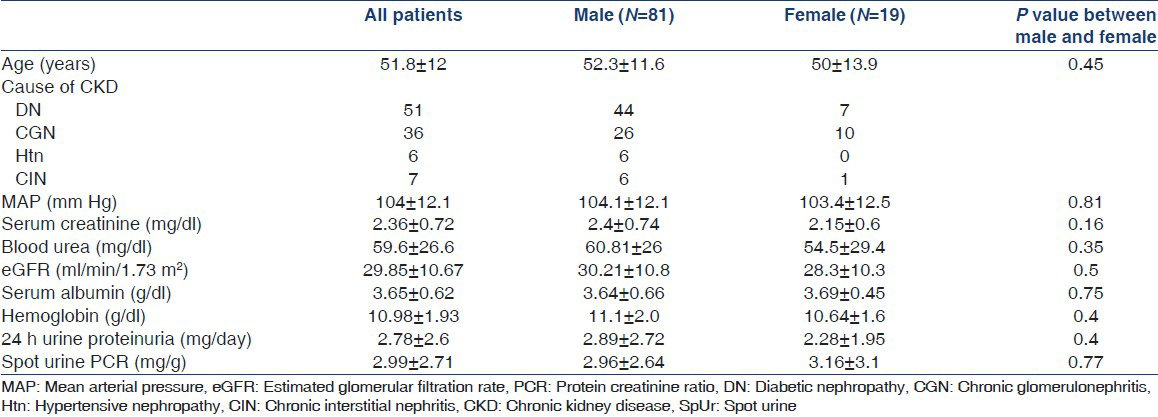
The mean age of the patients was 51.85 ± 12 years and 81% of the patients were male. Demographic and laboratory data are shown in Table 1. Table 2 shows results of anthropometric measurements in the study population. Correlation coefficient (r) between SpUr-PCR and 24 h-UP on a data transformed to a logarithmic scale was 0.86 (P < 0.001), 0.87 for male (P < 0.001) and 0.83 for female patients (P < 0.001). Correlation between the two methods of proteinuria measurement was better in CKD stage 3 (r = 0.86, P < 0.001) than in stage 4 (r = 0.71, P < 0.001). To assess the possibility of timing of urine sample introducing a bias, we analyzed the correlation between SpUr-PCR and urine PCR calculated from 24 h urine sample. The correlation between urine PCR calculated from 24 h urine collection sample and randomly collected urine sample was excellent (r on a data transformed to a logarithmic scale was 0.93 for both male and female, P < 0.001).

Among the three anthropometric surrogates for muscle mass, correlation with 24 h-UCr was better with MAC (r = 57) than with MAMC (r = 0.54) and MAMA (r = 0.53). Factors influencing 24 h-UCr were derived from linear regression analysis [Table 3], based on which an equation to estimate creatinine excretion in CKD patients was derived:

Estimated urinary creatinine excretion (mg/day) = (−533 + [261 in Males] + [31.7 × MAC in cm] + [28.9 × BMI] − [6 × Age]).
Table 4 shows the comparison of 24 h-UCr and that estimated by CG formula, formula proposed by Ix et al., and the formula derived from our study population. When a correction derived from the equation mentioned above was applied to the creatinine excretion for each patient, correlation on logarithmic transformed data between the two methods of proteinuria measurement improved (r = 0.88, P < 0.001; male, r = 0.9, P < 0.001; female, r = 0.82, P < 0.001).

Figure 1 shows scatter plots with goodness of fit analysis, showing linear relation between SpUr-PCR and 24 h-UP before (A) and after (B) addition of a correction factor. The r2 was 0.739 and 0.783 for all patients before and after correction for 24 h-UCr, respectively. The goodness of fit improved in men (r2 improved to 0.802 form 0.736), but not in women (r2 of 0.687 changed to 0.67) after correction for 24 h-UCr. Figure 2 shows Bland Altman plot showing level agreement between these measures before (A) and after (B) introduction of a correction factor. This showed that degree of agreement improved only in women after addition of correction (r2= 0.372 and 0.01 in women before and after correction), but not in men (r2= 0.002 and 0.041 before and after correction).

- Scatter plots with regression line on log transformed data comparing two methods of measurement of proteinuria before (a) and after (b) introduction of a correction factor for creatinine excretion. Interrupted line represents male (M), dotted line represents female (F) and uninterrupted line represents all patients

- Bland Altman plot showing goodness of fit between two measures of proteinuria before (a) and after (b) introduction of a correction factor for creatinine excretion. Interrupted line represents male (M), dotted line represents female (F) and uninterrupted line represents all patients
Correlation coefficient (r) between SpUr-PCR and 24 h-UP for patients with proteinuria < 1 g/day (N = 29) was 0.6 (P = 0.001), for proteinuria of 1-3.5 g/day (N = 41) was 0.73 (P < 0.001) and for proteinuria > 3.5 g/day (N = 30) was 0.35 (95% confidence interval (CI): −0.22-0.85, P = 0.062). After addition of correction, correlation between the two methods of proteinuria measurement improved significantly in the subgroup of patients with proteinuria >3.5 g/day (r = 0.45, 95% CI: 0.11-0.87, P = 0.013).
Discussion
We rigorously analyzed the accuracy of SpUr-PCR as a measure of proteinuria by correlation, precision, and degree of agreement and the findings of our study are summarized below. We found good correlation between 24 h-UP and SpUr-PCR in CKD stages 3 and 4 (r = 0.86). This correlation was better in men than in women. SpUr-PCR overestimated proteinuria, minimally in men and markedly in women. Correlation between the two methods of proteinuria measurement was better in CKD stage 3 than in stage 4. Overall precision of SpUr-PCR to predict 24 h-UP was good (r2= 0.739) and was better in men than in women. Bland Altman analysis showed a good agreement between the two methods of proteinuria measurement, especially, in men. In addition, correlation was very good in CKD patients with sub-nephrotic proteinuria; however, correlation as well as the degree of agreement was poor in patients with nephrotic range proteinuria. The few studies that have been carried out in CKD patients in general report a good correlation between SpUr-PCR and 24 h-UP.[24910111213] However, many find a wide CI and poorer correlation in nephrotic range proteinuria and at lower GFR.[10111213]
Creatinine excretion varies between different races and is the highest in the African race, followed by Caucasians and Asians.[619] Since variability in creatinine excretion is a major cause for error in proteinuria measurement by SpUr-PCR,[20] we felt that addition of a correction factor for creatinine excretion could improve the accuracy of the test. The only previous such an attempt was reported by Ginsberg et al.[4] They did not directly measure, but estimated the 24 h urine creatinine excretion by the formula proposed by CG.[16] After adding the correction factor for estimated creatinine excretion, they reported a good correlation between 24 h-UP and SpUr-PCR (r = 0.93); however, this did not further improve the accuracy of the test. In our study population, the formula to estimate creatinine excretion derived from Caucasian population such as CG formula and the formula recently proposed by Ix et al.,[17] markedly overestimated creatinine excretion; a finding similar to that reported by Jafar et al., in Pakistani population.[19] Creatinine excretion was close to 1 g/1.73 m2/day in men, but was markedly lower in women in our study population. The range of creatinine excretion in our study population was wide (350-1716 mg/day) and only 21% had creatinine excretion within 10% range (900-1100 mg/day) of expected 1 g/day. Recognition of the fact that urine creatinine excretion is markedly lower in south Asian population has significant implications for the clinical application of SpUr-PCR. In CKD population, linear regression analysis identified age, gender, MAC and BMI as factors that independently affect 24 h creatinine excretion and we derived a regression equation to predict daily creatinine excretion. This equation for estimating creatinine excretion has the advantage of being simple and determined based on easily measurable clinical parameters. Addition of correction factor for 24 h-UCr when applied to the measurement of SpUr-PCR (corrected SpUr-PCR) improved the performance of this test. This improved the accuracy in the subgroup with nephrotic range proteinuria, where a significant correlation was lacking prior to the introduction of correction factor. Furthermore, the introduction of correction factor improved the degree of agreement in women, but not the correlation. The utility of a correction for creatinine excretion in routine clinical practice is debatable. It may improve the accuracy of estimation in an individual especially when the creatinine excretion is markedly different from the expected 1 g/day and as shown in our study, in subsets of the population such as those with nephrotic range proteinuria and in women. However, to monitor serially the change in proteinuria in an individual patient over a period of time in clinical practice, the uncorrected SpUr-PCR may be sufficient since the factors that affect creatinine excretion in an individual generally remain the same over time.
Our study has several strengths. First, ours is the largest prospective study assessing the accuracy of SpUr-PCR specifically addressing CKD stages 3 and 4. Second, our study validates the use of SpUr-PCR for estimating proteinuria in advanced CKD in Asian Indians. Third, we derived an equation to estimate 24 h-UCr from regression analysis and added a correction factor for 24 h-UCr to improve the accuracy of SpUr-PCR. Though a marked variability in creatinine excretion among people of diverse age, gender and muscle mass was well-recognized, such a correction has not been attempted before.
Our study has a few limitations. First, the formula developed to estimate 24 h-UCr might not apply to CKD patients of a race other than South Asians. Secondly, it needs validation in a different set of Asian individuals. Third, the number of female CKD patients was small in our study and our results should be confirmed in larger female CKD populations.
Conclusions
SpUr-PCR accurately predicted 24 h-UP in our population of CKD stage 3 and 4. However, this test was less accurate in patients with nephrotic range proteinuria and women. Creatinine excretion was variable and was less than the presumed value of 1 g/1.73 m2/day. Adding a correction factor for creatinine excretion based on the formula derived from the regression equation improved correlation in the subgroup of patients with nephrotic range proteinuria and the degree of agreement in female patients, and hence may be used in expressing proteinuria measured by SpUr-PCR to improve its accuracy in them.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Cross sectional longitudinal study of spot morning urine protein: Creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. 1998;316:504-9.
- [Google Scholar]
- Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Kidney Int. 1998;53:1209-16.
- [Google Scholar]
- Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. 2001;55:436-47.
- [Google Scholar]
- Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543-6.
- [Google Scholar]
- Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478-94.
- [Google Scholar]
- Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol. 2002;13:1034-9.
- [Google Scholar]
- Use of protein: Creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin Chem. 2005;51:1577-86.
- [Google Scholar]
- Random spot urine protein/creatinine ratio is unreliable for estimating 24-hour proteinuria in individual systemic lupus erythematosus nephritis patients. Nephron Clin Pract. 2009;113:c177-82.
- [Google Scholar]
- Spot urine protein: Creatinine ratio versus 24 hour urine protein at various levels of GFR patients referred to a tertiary care hospital of Pakistan. J Pak Med Assoc. 2008;58:476-9.
- [Google Scholar]
- Is morning urinary protein/creatinine ratio a reliable estimator of 24-hour proteinuria in patients with glomerulonephritis and different levels of renal function? J Nephrol. 2004;17:666-72.
- [Google Scholar]
- The urine protein to creatinine ratio as a predictor of 24-hour urine protein excretion in type 1 diabetic patients with nephropathy. The Collaborative Study Group. Am J Kidney Dis. 1995;26:904-9.
- [Google Scholar]
- Comparison of 24-hour urinary protein and protein-to-creatinine ratio in the assessment of proteinuria. Saudi J Kidney Dis Transpl. 2009;20:443-7.
- [Google Scholar]
- Assessing proteinuria in chronic kidney disease: Protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. 2010;25:2991-6.
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184-91.
- [Google Scholar]
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National kidney foundation. Am J Kidney Dis. 2000;35(6 Suppl 2):S1-140.
- [Google Scholar]
- Serum creatinine as marker of kidney function in South Asians: A study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413-9.
- [Google Scholar]
- Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension. 2006;47:56-61.
- [Google Scholar]







