Translate this page into:
Successful renal transplantation from a brain-dead deceased donor with head injury, disseminated intravascular coagulation and deranged renal functions
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Deceased donors (DDs) with the brain death due to head injury are the major source of organs for transplantation. The incidence of post-head injury disseminated intravascular coagulation (DIC) ranges from 24% to 50%. Many centers do not accept organs from donors with DIC due to increased risk of primary graft non-function and/or high chances of morbidity/mortality. We performed two successful renal transplants from a DD with head injury with DIC and deranged renal function. One of the recipients developed transient thrombocytopenia, but there was no evidence of DIC or delayed graft functions in either of the recipients. Over a follow-up of 1 month, both are doing well with stable graft function and hematological profile. Thus, a carefully selected DD with severe DIC even with deranged renal function is not a contraindication for organ donation if other risk factors for primary non-function are excluded. This approach will also help in overcoming organ shortage.
Keywords
Deceased donor
disseminated intravascular coagulation
renal transplant
Introduction
Deceased donors (DDs) with the brain death due to head injury are the major source of organs for transplantation. Recent studies mention incidence of post-head injury disseminated intravascular coagulation (DIC) ranging from 24% to 50%.[123] Many centers hesitate in accepting organs from donors with DIC due to increased risk of primary graft non-function and/or high chances of morbidity/mortality.
In India, about 61% of stage V chronic kidney disease (CKD) patients are not on any form of renal replacement therapy and only 2% are prepared for renal transplantation.[4] There is a wide gap between the demand and supply of organs for patients with end organ failure and potential chances of getting a deceased donor (DD) organ in the present scenario are dismal. To reduce this disparity, various strategies are being implemented to expand the DD pool. An additional approach that could be considered is accepting DD with head injury with disseminated intravascular coagulation (DIC) for organ donation since, DD with traumatic head injury are the most common source of DD organ transplantation. Whether to consider a DD with DIC is controversial since some studies have shown increased incidence of primary non-function in transplanted kidneys while others have shown good long-term outcome, but initial delayed graft function.[567]
We present two successful renal transplants (RTx) from a brain-dead DD with head injury with extensive DIC and deranged renal function.
Case Report
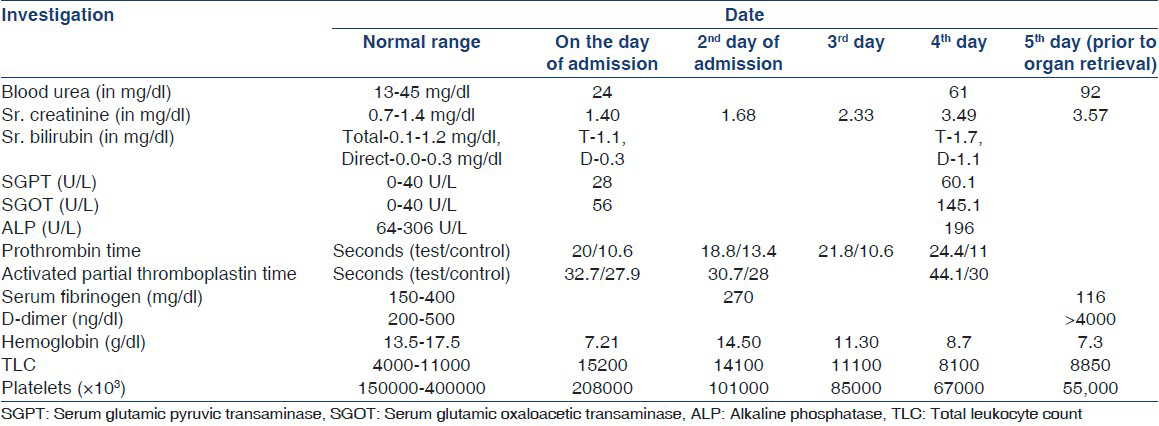
A 19-year-old male patient with head injury following a road traffic accident was brought in emergency to a private trauma care hospital. On admission, he was found to be comatose, had fractured right tibia and left femur. On day of admission, his renal function and liver function tests were normal with serum creatinine (SCr), 1.40 mg/dL. However, he had deranged coagulation profile with activated partial thromboplastin time: 32.7 s, (control: 27.9 s), Prothrombin time: 20 s (control: 10.6 s) and international normalized ratio was 1.89. His hemoglobin was 7.21 g/dL, total leukocyte count 1.52 × 103/μl and platelet count was 2.08 × 105/ μl. He had hypotension and fall in hemoglobin level; hence, he was transfused 4 units of whole blood and started on vasopressors dopamine at 10 μg/kg/min and noradrenaline at 10 μg/ min. Blood pressure was maintained around 110-120 systolic and 80-90 mm of Hg diastolic. On 2nd day, he developed extensive petechiae and purpura all over the body and oozing of blood through wounds hence 6 units of fresh frozen plasma were transfused. However, he further deteriorated with increase in purpuric spots, further derangement in coagulation profile, decreasing platelet count and Hemoglobin level, low Serum fibrinogen level and D-DIMER >4000 ng/ml suggestive of extensive DIC. Detaillaboratory parameters of the donor are shown in Table 1. Computed tomography brain was suggestive of changes of diffuse hypoxia. Electroencephalogram was suggestive of alpha alpha coma. Patient was declared brain-dead by neurophysician and neurosurgeon at the interval of 6 h. Ultrasonography of the abdomen showed normal sized kidneys. Blood and urine culture were sterile. The relatives were explained about organ donation. Since, they agreed he was shifted to our institute. His renal function was deranged with SCr, 3.57 mg/dL and blood urea 92 mg/dL. His hemoglobin had dropped to 7.3 g/ dL and platelet count was 5.5 × 104/μl. Blood pressure was 100/70 mmHg on dopamine and noradrenalin support. Both kidneys were harvested and transplanted in two recipients with favorable complement-dependent lymphocytotoxicity cross-match.
Recipient 1-was a 25-year-old male on maintenance hemodialysis for, 55 months. He received induction therapy with rabbit anti-thymocyte globulin (r-ATG) 1.5 mg/kg and 3 doses of methyl prednisolone 500 mg/ day. His hematological, coagulation and renal function profile remained within normal range throughout the post-operative period and follow-up period of 1 month. SCR normalized on 5th post-operative day to 1.22 mg/ dL and he was discharged on 7th post-operative day with SCR, 1.18 mg/dL on maintenance immunosuppression of Tacrolimus 0.08 mg/day, Mycophenolate sodium 720 mg twice daily and Prednisolone, 20 mg/day. There was no evidence of graft dysfunction throughout the follow-up period of 1 month.
Recipient 2 was a 30-year-old female with post-partum cortical necrosis. She was on maintenance hemodialysis since 44 months. She received the same induction and maintenance immunosuppression therapy as recipient 1. On first post-operative day, her platelet count decreased from 1.44 × 105/μl to 9.5 × 104/μl. However, it recovered gradually and normalized on 4th post-operative day without any treatment. Coagulation profile performed performed on 1st and 4th post-operative day was normal and there was no evidence of bleeding diathesis. SCR normalized on 8th post-operative day to 1.27 mg/dL. She was discharged on 12th post-operative day with SCR of 0.87 mg/dL with same maintenance immunosuppression as that of recipient 1. Demographic profile and laboratory parameters of both recipients are shown in Table 2. Sr. Tacrolimus level of both transplant recipients in immediate post-transplant period and throughout the follow-up period of 1 month was maintained in the range of 7-10 ng/ml.

Discussion
DIC is common in DD with head injury with incidence of 59% in open head trauma, 43% in combined open and closed head trauma and 37% in closed head trauma.[8] Mechanisms responsible for DIC are activation of the coagulation cascade through release of brain tissue thromboplastin, inflammatory cytokines, tissue factor activation and exposure of phospholipids to circulating blood.[910] DIC causes occlusion of small and medium sized vessels due to the formation of fibrin thrombi.[11] Many centers still hesitate to accept organs from DD with DIC due to reported incidence of primary non-function. In one study, fibrin thrombi present in renal biopsy at 1 h post-transplant was not present in biopsy done at 7 days and 6months post-transplant. This suggested that fibrin thrombi can be lysed by recipient fibrinolytic system.[1213141516] Recipients of grafts with donor thrombi were more likely to exhibit delayed graft function; however, graft function and survival at 1 and 2 years post-transplant was good suggesting that the presence of donor microvascular thrombosis doesn’t n’t portend poor outcome in RTx.[17]
Some studies suggest that the presence of good renal function and renal biopsy is necessary prior to transplantation to exclude renal cortical necrosis due to microvascular thrombosis in DIC, but other reports negate this view.[181920] Pre-transplant renal biopsy is not a good method to guide organ allocation in cases of donor DIC because of false positive and false negative results and also inability of renal biopsy to predict reversibility of fibrin thrombi by glomerular fibrinolytic system after transplantation.[21222324252627] One study reported increased incidence of post-transplant thrombocytopenia in recipients of kidney from DIC positive donors. It also noted that there was increased incidence of delayed graft function in recipients who had post-transplant thrombocytopenia, but long-term graft survival was good.[28] Recipient thrombocytopenia may be an early sign of intrarenal clotting due to DIC; thus, may contribute to delayed graft function or slow graft function.[282930] There are some case reports, which showed that even presence of severe DIC with thrombotic microangiopathy on renal biopsy and renal function impairment in DD is not a reason for excluding organ from DIC positive donor.[31]
We selected this donor for organ donation because of his young age and since, there was no any major illness in the past; also he had good urine output prior to transplant. We proceeded without performing pre-transplant renal biopsy. One of our recipients developed transient thrombocytopenia, but there was no clinical or laboratory evidence of DIC and also there was no evidence of delayed graft function or slow graft functions in either recipient. We attribute this transient thrombocytopenia to r-ATG given for induction.
Conclusion
To conclude, a carefully selected DD with head injury and DIC even with deranged renal function may not be a contraindication for organ donation if other risk factors for primary non-function are excluded.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury: Analysis of 242 cases. Br J Neurosurg. 2011;25:363-8.
- [Google Scholar]
- Coagulopathy in acute head injury: A study of its role as a prognostic indicator. Br J Neurosurg. 1997;11:398-404.
- [Google Scholar]
- Coagulopathy after isolated severe traumatic brain injury in children. J Trauma. 2011;71:1205-10.
- [Google Scholar]
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Donor disseminated intravascular coagulation (DIC), intraglomerular fibrin deposition and subsequent graft function. Kidney Int. 1978;13:432.
- [Google Scholar]
- Disseminated intravascular coagulation in cadaver kidney donors. Transplant Proc. 1986;18:469.
- [Google Scholar]
- Disseminated intravascular coagulation in cadaveric organ doners, incidence and effect on renal transplantation. Transplantation. 1993;55:2-442.
- [Google Scholar]
- Disseminated intravascular coagulation: Old disease, new hope. BMJ. 2003;327:974-7.
- [Google Scholar]
- Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533-42.
- [Google Scholar]
- Disseminated intravascular coagulation in multiorgan donors. Transplant Proc. 1992;24:33.
- [Google Scholar]
- Hemostasis and fibrinolysis in renal transplantation. Semin Thromb Hemost. 1989;15:88-9.
- [Google Scholar]
- Return of function in the thrombosed kidney after transplantation. Nephron. 1977;19:146-52.
- [Google Scholar]
- Kidney retrieval from cadaver donors with disseminated intravascular coagulopathy. Curr Surg. 1989;46:6-9.
- [Google Scholar]
- Prognostic significance of microvascular thrombosis in donor kidney allograft biopsies. Transplantation. 2003;75:1847-52.
- [Google Scholar]
- Acute cortical necrosis. Case report and review of the literature. Am J Med. 1974;56:110-8.
- [Google Scholar]
- Disseminated intravascular coagulation (DIC): An approach. Am J Med. 1972;52:679-89.
- [Google Scholar]
- Pathology of disseminated intravascular coagulation (DIC). Analysis of 26 cases. Hum Pathol. 1972;3:327-43.
- [Google Scholar]
- Clinicopathological correlations of disseminated intravascular coagulation in patients with head injury. Neurosurgery. 1984;15:34-42.
- [Google Scholar]
- Studies on the nature of fibrinoid in the collagen diseases. Am J Pathol. 1957;33:55-77.
- [Google Scholar]
- Variations in the staining characteristics of human fibrin. Am J Pathol. 1957;33:267-83.
- [Google Scholar]
- Microscopic intrarenal particles after pulsatile machine preservation do not adversely affect outcomes after renal transplantation. Transplant Proc. 2006;38:3384-7.
- [Google Scholar]
- Implications of donor disseminated intravascular coagulation on kidney allograft recipients. Clin J Am Soc Nephrol. 2011;6:1160-7.
- [Google Scholar]
- The pathogenesis and management of disseminated intravascular coagulation. Clin Adv Hematol Oncol. 2006;4:919-26.
- [Google Scholar]
- Does disseminated intravascular coagulation lead to multiple organ failure? Crit Care Clin. 2005;21:469-77.
- [Google Scholar]
- Kidney donor with severe disseminated intravascular coagulation: Transplantation however successful. Ned Tijdschr Geneeskd. 2009;153:B418.
- [Google Scholar]







