Translate this page into:
Outcome of live and deceased donor renal transplantation in patients aged ≥55 years: A single-center experience
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal transplantation (RTx) has now become an accepted therapeutic modality of choice for elderly ESRD patients. This single-center study was undertaken to evaluate the outcome of RTx in ESRD patients ≥55 years. A total of 103 patients underwent RTx 79 living related living donors [LD], 24 deceased donors [DD]) at our center. Post-transplant immunosuppression consisted of calcineurin inhibitor–based regimen. The mean donor age was 58.3 years in the LD group and 59.5 years in the DD group. Male recipients constituted 92% in LD and 75% in DD group. In living donor renal transplantation, 1- and 5-year patient survival was 93% and 83.3% respectively and death-censored graft survival was 97.3% and 92.5% respectively. There were 12.6% biopsy proven acute rejection (BPAR) episodes and 12.6% patients were lost, mainly due to infections. In deceased donor renal transplantation, 1- and 5-year patient survival was 79.1% and 74.5% respectively and death-censored graft survival was 95.8% and 85.1% respectively. There were 12.5% BPAR episodes and 25% of patients were lost, mainly due to infections. RTx in ESRD (≥55 years) patients has acceptable patient and graft survival if found to have cardiac fitness and therefore should be encouraged.
Keywords
Deceased donor renal transplantation
end-stage renal disease
live donor renal transplantation
transplantation in elderly
Introduction
The end-stage renal disease (ESRD) population is ageing.[123] Complications of hemodialysis occur with increased frequency in elderly ESRD patients.[2] It remains unclear whether the possible benefits of renal transplantation (RTx) are sufficient to advocate transplantation over dialysis in elderly ESRD. Additional aspects of RTx that may differ in an elderly patient include ethics of transplantation, pre-transplant evaluation, mechanisms of graft loss and death and degree and type of immunosuppressive therapy. Studies at various centers have proved that eligible elderly patients can also benefit from RTx like young patients when compared with their management on dialysis.[123456789] However, there is a paucity of data on the outcome of RTx in older patients with ESRD from developing countries.[3] We present our 6 years’ experience with RTx in ESRD patients who received a transplant at ≥55 years.
Materials and Methods
We analyzed the outcome of 103 RTx (79 living-related donor (LRD) and 24 deceased donor (DD) in ESRD patients ≥ 55 years old at the time of tranbsplantation between 2005 and 2010. Approval by the Internal Review Board and written informed consent from all patients were obtained.
Recipient selection
All ESRD patients were viewed as initial transplant candidates. Patients with untreated current infections, unstable/active heart disease (patients requiring surgical cardiac intervention/revascularization therapy), active malignancy with short life expectancy, chronic illness with life expectancy of less than 1 year, poorly controlled psychosis, active substance abuse and active malignancy were excluded. Patients and caregivers were counseled that in eligible patients RTx improves survival even in older ESRD patients and it will be cost-effective as compared with maintenance dialysis. A team of nephrologists, urologists, anesthetics, pathologists and transplant co-coordinator were involved in the pre-transplant evaluation. Transplant patients were selected on the basis of their cardiac fitness, an estimated life expectancy of at least 5 years based on the clinical evaluation and laboratory reports, absence of a major contraindication to immunosuppressive therapy and an estimated low perioperative risk. Those accepted for RTx were encouraged to find LRD. If potential LD were unavailable, DDRTx was performed. Patients were reviewed every 2 months while on the waiting list for transplantation. None of them had preemptive transplantation.
Pre-transplant cardiac evaluation
It included history, physical examination, electrocardiogram, chest radiograph and echocardiogram. Cardiac catheterization was performed in patients with symptoms and/or signs consistent with coronary artery disease, history of myocardial infarction and/or unstable angina, unless they recently underwent successful revascularization. The decision to proceed with angioplasty and/or surgery was based on the findings of catheterization. Coronary angiogram was performed among all diabetic >55 years old potential recipients. If positive, the decision to proceed with angioplasty or surgery was usually made in conjunction with the patient's cardiologist.
Recipient selection in deceased donor renal transplantation (DDRTx)
According to our institutional rules, the grafts were allocated according to an old-for-old system and waiting time. Human leukocyte antigen (HLA) matching was not used as a criterion for recipient selection. Immunologically high-risk recipients or those with vascular access problems were also given priority.
A wedge biopsy was obtained at the time of organ procurement from older donors with a history of hypertension (HTN) or diabetes mellitus, cerebrovascular accident (CVA) as the cause of death, elevated serum creatinine. Frozen section was studied within 1 h for percentage of glomerulosclerosis. If it was <15% a single kidney transplant was performed; if it was 15-50%, a dual kidney transplant was performed.
Immunosuppressive regimen
All patients received induction immunosuppressive therapy with methylprednisolone (500 mg intravenously × 3 days) ± rabbit-antithymocyte globulin (r-ATG) (1.5 mg/kg, single dose) in high immunologic risk group. Maintenance immunosuppression consisted of prednisolone (20 mg/day, tapered to 5-10 mg/day at 1-3 months post-transplant and continued thereafter), calcineurin inhibitors (CNI) (cyclosporine [CsA] [3-5 mg/kg/day] or tacrolimus [TaC], [0.06-0.08 mg/kg/day]) and/or mycophenolate mofetil (MMF) (1.5-2 g/day) or azathioprine [AZA] 1-2 mg/kg/day. CsA was preferred in patients who were HCV positive/diabetics. TaC was used in others. The doses of AZA and MMF were adjusted according to complete blood counts. The doses of CNI were adjusted based on the serum trough levels (C0), measured by fluorescence polarization immunoassay technology during the first 2-3 months; subsequently adjustments were made only in case of graft dysfunction. This decision was due to the financial constraints. CsA dosing was adjusted to achieve target C0 concentration of 200-250 ng/mL during the first 2-3 months post-transplantation, 100-200 ng/mL 3-6 months post-transplantation and ~100 ng/mL thereafter. TaC dosing was adjusted to achieve target T0 concentrations of 4-7 ng/mL. All patients received prophylaxis against cytomegalovirus (CMV), fungal and pneumocystis jiroveci pneumonia infection. Graft biopsy was performed in cases of acute graft dysfunction, diagnosed as per the modified Banff classification and treated according to standard guidelines.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 12.0, IBM SPSS Inc., Chicago Ill). Continuous variables were compared using Student t-test. Chi-square test of fisher exact test was used to assess the effect of change in differences in categorical variables. Survivals were examined using Kaplan-Meier analysis and compared using the log-rank test. P <0.05 was considered statistically significant.
Results
Out of 1794 RTx performed in our center between 2005 and 2010, 103 (5.7%) were for elderly ESRD patients. There were 79 LD (Group 1) and 24 DDRTx recipients (Group 2).
Recipient and donor characteristics in living donor renal transplantation (LDRTx)
There were 73 males and 6 females, with a mean age of 58.3 ± 3.96 (range: 55-73) years. Original disease leading to ESRD was chronic glomerulonephritis (CGN) (n = 24), diabetic nephropathy (DN) (n = 33), HTN (n = 9), autosomal dominant polycystic kidney disease (ADPKD) (n = 5) and others (n = 8). Mean donor age was 42.03 ± 12.5 (range: 20-55) years, 33 were men and 46 were women. LDs were spouses (n = 34), siblings (n = 14), off-springs (n = 17) and extended family members (n = 14), with mean HLA match of 2 ± 1.4. The mean dialysis duration before RTx was 12 ± 4.5 months. Immunosuppressive regimen included CsA (42%), TaC (58%).
Post-transplant outcome data in DDRTx
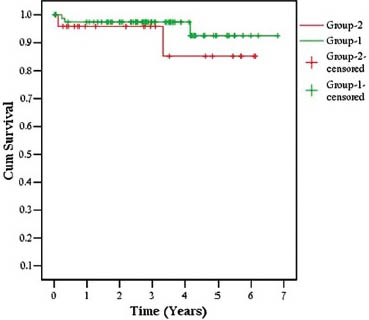
Over a mean follow-up of 3.0 ± 1.5 years, 1-and 5-year patient survivals were 93% and 83.3% and death-censored graft survival was 97.3% and 92.5% for 1 and 5 years, respectively. A total of 12.6% (n = 10) patients were lost, mainly due to infections (n = 8) (CMV disease [n = 1], tuberculosis [n = 1], fungal infection [n = 1], pneumonia with acute respiratory distress [n = 3], hepatic encephalopathy secondary to chronic viral hepatitis [n = 1]), CVAs (n = 1), cardiovascular disease (CVD) (n = 1) and post-transplant lymphoproliferative disorder (n = 1)). There were 12.6% (n = 10) biopsy proven acute rejection (BPAR) episodes, out of which 5% (n = 4) were acute B-cell mediated rejections acute humoral rejection (AHR), 1.2% (n = 1) acute T-cell mediated rejections (ATR), 6.3% (n = 5) were combined acute T + B-cell mediated rejections and 1.2% (n = 1) had unexplained interstitial fibrosis with tubular atrophy (IFTA). Most of them (n = 8) recovered after anti-rejection therapy (ART); however two patients died from bacterial or viral infections within 6 months of ART, whereas IFTA eventually led to graft loss. Survival rates are shown in Kaplan-Meier curves Figure 1 (Group 1 LDs and Group 2 DDs) and Figure 2.

- Kaplan-Meier patient survival curves in living versus deceased donors
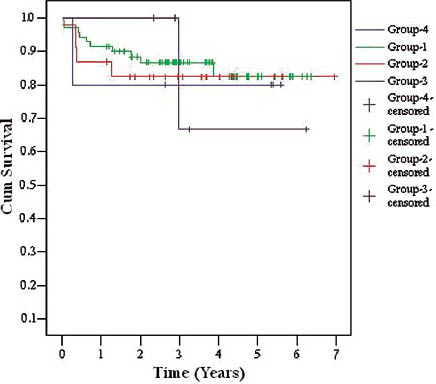
- Kaplan-Meier death censored graft survival curves in living versus deceased donors
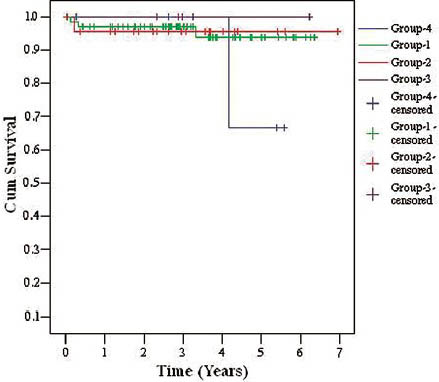
- Kaplan-Meier patient survival curves in different age subgroups
- Kaplan-Meier death censored graft survival curves in different age subgroups
Recipient and donor characteristics in DDRTx
There were 18 male and 6 female recipients, with a mean age of 59.5 ± 5.34 (range: 55-76) years. Original disease leading to ESRD were CGN (n = 5), DN (n = 8), HTN (n = 5), ADPKD (n = 3) and others (n = 3). Mean donor age was 50.3 ± 20.3 (range: 20-89) years, 15 were men and nine were women. There were three dual kidney transplants and five were non-heart-beating donations. Data on HLA matching were not available for analysis in this group. The mean dialysis duration before RTx was 21.5 ± 5.5 months. Immunosuppressive regimen included CsA (50%) and Tac (50%).
Post-transplant outcome data in DDRTx
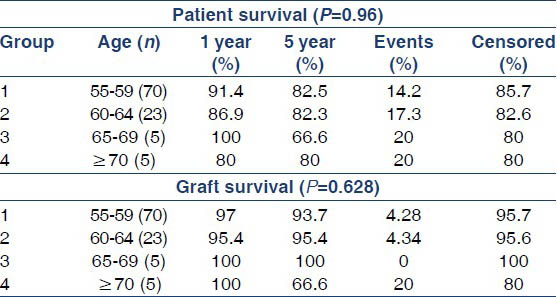
Over a mean follow-up of 3.16 ± 1.88 years, 1- and 5-year patient survival was 79.1% and 74.5%, respectively and death-censored graft survival was 95.8% and 85.1% for 1 and 5 years, respectively. Delayed graft function was observed in 37.5% (n = 9) patients. A total of 25% (n = 6) patients were lost, mainly due to infections (n = 5) (CMV disease (n = 1), tuberculosis (n = 1), fungal infection (n = 1), pneumonia with acute respiratory distress (n = 2) and CVA (n = 1). There were 12.5% (n = 3) BPAR, out of which 4.1% (n = 1) AHR, 4.1% (n = 1) ATR, 4.1% (n = 1) had combined AHR + ATR and 4.1% (n = 1) had IFTA. Two patients recovered and two patients succumbed to infections within 6 months of ART. There was no significant difference between the patient (P = 0.96) and the graft survival (P = 0.628) in different age subgroups of patients as shown in Table 1, Figure 2a and b (Group 1: recipient age 55-59 years, Group 2: recipient age 60-64 years, Group 3: recipient age 65-69 years, Group 4: recipient age ≥70 years).
Discussion
Chronic kidney disease (CKD) is becoming a major public health problem world-wide. It is associated with major complications including increased risk of mortality, ESRD, accelerated CVD, mineral and bone disease, adverse metabolic and nutritional consequences and infections. Mortality from CVD is estimated to be at least 8- to 10-fold higher in CKD subjects compared with non-CKD subjects. More than 40% of deaths in patients on dialysis are the result of cardiovascular causes.[101112]
Clinicians have been reluctant to transplant kidneys into elderly patients for a number of reasons. These include limited life expectancy of the elderly in general and the elderly patient with ESRD in particular and poor results of RTx with immunosuppressive regimens in the pre-CsA era.[13] In the current scenario, both patient and graft 5 year survival amongst the elderly has markedly improved negating the misconception that age alone negatively influences successful transplantation.[1415] At present, the most important reason for the reluctance to transplant elderly individuals is the gap between demand and supply for organs. Hence we advocate age-matched transplants, thus giving a chance of RTx to elderly and also increasing the donor pool with marginal donors.[3] According to Canadian Society of Transplantation guidelines, advanced age per se is not a contraindication to RTx.[16] The decision for transplantation must be made in the best interest of the patient and should be based on medical and surgical grounds.
Most transplant centers consider elderly transplant patients to be at least 65-70 years old.[1] This criterion may not be applicable for developing countries like India since our patients usually do not reach this age without RTx due to the local health and hygienic conditions as well-economic constraints. Our ESRD patients underwent RTx within 5 years of diagnosis or they die with or without dialysis. The mean age of our CKD registry patients is 50 years.[17] Hence, we have considered patients ≥55 years as elderly ESRD in our study. RTx is a cost-effective form of renal replacement therapy for ESRD population versus maintenance dialysis.[18]
The objective of the present study is to evaluate the efficacy and survival of RTx in ESRD patients (≥55 years). The survival is diminished by waiting for prolonged time on dialysis and access to transplantation diminishes with time due to development and/or progression of CVD and other illnesses.
Older transplant recipients experience more infectious complications and less acute rejection, but the risk of transplant loss from rejection is increased compared with younger patients. These immunologic issues, along with the fact that older patients often are excluded from transplant trials, have made selecting an ideal immunosuppressive regimen challenging.[19]
A comprehensive pre-operative evaluation and modified immunosuppressive therapy for elderly transplant recipient is imperative to decrease morbidity and mortality following transplantation due to CVD and infection.[20212223] Our study showed reduced mortality from CVD, due to careful pre-transplant cardiac evaluation.
The possible mechanisms to explain the decreased incidence of acute rejection in the elderly include impaired co-stimulatory pathway of allorecognition, alteration in phenotypes and functions of T-cells, reduced number of naýve T-cells, dysfunctional memory cells, increased sensitivity to immunosuppression, reduced T-cell receptors and defective T-cell signaling, increased T suppressor cells and altered cytokine profile.[2021222324]
The high infection rate despite judicious use of immunosuppressive agents and infection prophylaxis is not specific to elderly patients, but it is common in the transplantation setting in our country. Unhygienic living conditions, delayed presentation and diagnosis, tropical climate, limited availability and high cost of diagnostic tools, r-ATG induction and financial constraints for treatment in the majority of patients are usually the reasons for incidence of high infection rate in RTx patients in developing countries.[25262728]
RTx provides better quality-of-life and is also cost-effective than dialysis especially in elderly patients. However, few studies have shown a survival advantage with age stratification in transplantation among the elderly, including recipients of extended criteria donor (ECD) kidneys.[1342930313233] We have attempted the correlation of age-stratification with survival analysis in the present study.
Renal allograft and patient survivals in the elderly transplant recipient are currently excellent. With present therapy, patient survival at 1, 5 and 10 years is approximately 80-90%, 70% and 50%, respectively.[13131415343536] In a study of 5667 waitlist patients older than 70 years of age, the risk of death was significantly lower with DDRTx versus remaining on the wait list.[1] This benefit extended to those who received an ECD kidney. One study from India has shown 1 year death-censored graft survival of 93.1% and for next 4 years this survival was reported as 91.2%. In this study patient survival was 92.5% in 1st year, 90.7% in the next 2 years and 89.2% in 4th year. BPAR rate was 28.7% with the majority of deaths due to infections and CVD.[8] There is no national renal transplant registry data in India to evaluate the survival advantage of elderly RTx patients. Hence, the present study will be helpful in future to evaluate RTx in ESRD (≥55 years) population of developing countries.
We have previously reported long-term outcome of 1523 LD renal allograft recipients in young.[37] The outcome in our older ESRD patients (≥55 years) is comparable with our young ESRD patients.
Limitations
Of our study are a small sample size and lack of direct comparison with waiting list patients on MHD. This study includes both live (variable HLA matches) and DD kidney (non-heart beating and dual kidney) transplant recipients. The induction and maintenance immunosuppression is variable.
Randomized trials are necessary to better define the optimal immunosuppressive regimen for elderly transplant recipients.
Conclusion
In eligible patients, RTx for ESRD (≥55 years) has acceptable patient and graft survival and hence should be encouraged. The patient must fulfill the medical criteria for acceptance for transplantation.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Renal transplantation in elderly patients older than 70 years of age: Results from the scientific registry of transplant recipients. Transplantation. 2007;83:1069-74.
- [Google Scholar]
- Geriatric nephrology: A paradigm shift in the approach to renal replacement therapy. Adv Chronic Kidney Dis. 2011;18:412-9.
- [Google Scholar]
- Renal transplantation and the elderly patient. Last literature review version 193: September. 2011. Available from: http://www.uptodate.com
- [Google Scholar]
- How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945-51.
- [Google Scholar]
- Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States. Int Urol Nephrol. 2005;37:641-8.
- [Google Scholar]
- Renal transplantation in the elderly: Does patient age determine the results? Age Ageing. 2005;34:583-7.
- [Google Scholar]
- Graft and quality of life outcomes in older recipients of a kidney transplant. Exp Clin Transplant. 2003;1:69-72.
- [Google Scholar]
- Renal transplantation in the elderly: South Indian experience. Int Urol Nephrol. 2011;43:265-71.
- [Google Scholar]
- Transplantation: Optimizing outcomes in elderly kidney transplant recipients. Nat Rev Nephrol. 2013;9:382-4.
- [Google Scholar]
- The impact of CKD identification in large countries: The burden of illness. Nephrol Dial Transplant. 2012;27(Suppl 3):iii32-8.
- [Google Scholar]
- Cardiovascular disease: The price of a QALY-cost-effectiveness of statins in CKD. Nat Rev Nephrol. 2013;9:377-9.
- [Google Scholar]
- Chronic kidney disease: Global dimension and perspectives. Lancet 2013 Epub ahead of print
- [Google Scholar]
- Renal replacement therapies in the elderly: Part II. Renal transplantation. Am J Kidney Dis. 1994;23:1-15.
- [Google Scholar]
- The 2008 SRTR report on the state of transplantation. Available from: http://www.ustransplant.org/annual_reports
- [Google Scholar]
- Patient and graft survival in older kidney transplant recipients: Does age matter? J Am Soc Nephrol. 2004;15:1052-60.
- [Google Scholar]
- Canadian Society of Transplantation: Consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:S1-25.
- [Google Scholar]
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Evolution of renal transplantation in India over the last four decades. NDT Plus. 2010;3:203-7.
- [Google Scholar]
- Induction of allograft tolerance through costimulatory blockade: First selection of drugs in vitro. Transpl Immunol. 2003;11:215-22.
- [Google Scholar]
- Early lymphocyte activation in elderly humans: Impaired T and T-dependent B cell responses. Exp Gerontol. 1999;34:217-29.
- [Google Scholar]
- Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20:171-8.
- [Google Scholar]
- Deceased donor organ transplantation: A single center experience. Indian J Nephrol. 2011;21:182-5.
- [Google Scholar]
- Outcome of renal transplantation in patients with diabetic nephropathy: A single-center experience. Int Urol Nephrol. 2011;43:535-41.
- [Google Scholar]
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69:794-9.
- [Google Scholar]
- “Old-for-old” cadaveric renal transplantation: Surgical findings, perioperative complications and outcome. Eur Urol. 2003;44:701-8.
- [Google Scholar]
- Survival benefits of kidney transplantation with expanded criteria deceased donors in patients aged 60 years and over. Transplantation. 2007;84:1618-24.
- [Google Scholar]
- Renal transplantation with cyclosporine in the elderly population. Transplant Proc. 1991;23:1749-52.
- [Google Scholar]
- Long-term outcomes of renal transplants from spousal and living-related and other living-unrelated donors: A single center experience. J Assoc Physicians India. 2012;60:24-7.
- [Google Scholar]







