Translate this page into:
Protective effects of Cornus mas fruit extract on carbon tetrachloride induced nephrotoxicity in rats
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oxidative damage is implicated in the pathogenesis of kidney injury. Cornus mas is used for in renal aliments traditionally in Iran. The present study was aimed to investigate the antioxidant activity of C. mas fruit extract (CMFE) on carbon tetrachloride (CCl4) treated oxidative stress in Wistar albino rats. Forty two male albino rats were divided into seven groups. Group I served as a sham; Group II served as a normal control; Group III served as a toxic control, with CCl4 (1 ml/kg body weight; 80% in olive oil); Groups IV and V received CMFE at doses of 300 and 700 mg/kg before CCl4 injection; Groups VI and VII received extract at same doses orally at 2, 6, 12, 24 and 48 h after CCl4 intoxication. CCl4 injection produced a significant rise in serum markers of oxidative stress and lipid peroxidation product malondialdehyde along with the reduction of antioxidant enzymes such as superoxide dismuta, catalase and glutathion peroxidase. Serum creatinine, urea and uric acid concentrations were increased whereas level of protein and albumin were reduced. Treatment of rats with different doses of fruit extract (300 and 700 mg/kg) significantly (P < 0.05) ameliorated the alterations induced with CCl4 in lipid peroxidation, antioxidant defenses, biochemical and renal lesions. Based on these results, we conclude that CMFE protects kidney from oxidative stress induced by CCl4.
Keywords
Carbon tetrachloride
Cornus mas
lipid paroxidation
nephrotoxicity
oxidative stress
Introduction
Exposure to various organic compounds including a number of environmental pollutants and drugs can cause cellular damage through generation of reactive oxygen species (ROS). Carbon tetrachloride (CCl4), a clear, colorless, volatile, heavy and nonflammable liquid, causes free radical generation and causes kidney injury in rats.[12] Free radicals induce lipid peroxidation and can damage cell membranes.[3] The average daily intake of CCl4 for the general population is estimated to be 0.1 μg. Kidney failure is frequently reported in fatal poisoning.[45] Furthermore, based on results of animal studies, the US Environmental Protection Agency has classified CCl4 as a Group B2, probable human carcinogen.[56] In spite of the fact that the harmful effects of CCl4 are obvious, this compound is still used as a solvent for oils, fats, lacquers, varnishes, rubber waxes and resins and as a starting material in the production of a number of organic compounds.[67]
It has been established that trichloromethyl (CCl3) radical and Cl are formed as a result of the metabolic conversion of CCl4 by cytochrome P450, which in turn, initiate lipid peroxidation process.[8910]
Among horticultural crops, fruits are sources of diverse nutrient and non-nutrient molecules, which display antioxidant properties,[11] and can protect the human body against oxidant damage.
Cornus mas, known as the European and Asiatic Cornelian Cherry, has been used for the treatment of diarrhea, intestinal inflammation, fever and malaria.[12] Furthermore, it has been mentioned for the treatment of kidney stones, kidney treatment and bladder infections in traditional system of medicine in Iran. Chemical characterization of C. mas fruit has shown that it is a rich source of phenolic and antioxidant, anthocyanins and flavonoids compounds.[1213] Despite the favorable ethnopharmacological properties, its protective effect against CCl4 nephrotoxicity has not been explored. In the present study, we investigated the effects of C. mas fruit on oxidative stress parameters in CCl4-induced nephrotoxicity in rats.
Materials and Methods
Chemicals
Trichloroacetic acid (TCA) and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich Chemical Co. Ltd. (USA). Thiobarbituric acid (TBA) and CCl4 were obtained from Merck Co. (Germany). Assay kits for the estimation of creatinine, urea and acid uric were purchased from Pars Azma (Iran) and all other chemicals used were of analytical grade were obtained from either Sigma-Aldrich or Merck (Germany).
Plant material
C. mas plant and its fruits were authenticated by the Botany Department of Tabriz University, Iran and obtained from suburbs of Arasbaran protected jungle (East Azerbaijan, Iran) at the end of spring 2012. The fruits were air-dried, protected from direct sunlight and powdered. The powder was kept in a closed container at 8°C.
Extraction
A total of 500 g of powder was extracted with a mixture of methanol: water (7:3) at 25°C. The solvent was completely removed by rotary vacuum evaporator at 50°C. C. mas fruit extract (CMFE) was frozen at − 20°C until use. The yield of the extract was 50% with reference to dry starting material.
Toxicity study
For toxicity studies, groups of 10 mice were administered (i.g.) the test compounds in the doses 100-1650 mg/kg. The LD50 (LD50 = 1270) was determined using the graphical methods of Litchfield and Wilcoxon.[14] Two different doses were selected to evaluate the dose dependent effect of CMFE on CCl4-induced nephrotoxicity.
Furthermore, based on previous studies about evaluation of toxicity effects of CCl4 like Zargar (2010), desired dose of CCl4 was selected.
Animals and treatment
Male albino rats of Wistar strain (250-300 g) were purchased from Pasteur Institute (Tehran, Iran). The animals were housed in polypropylene cages in a temperature-controlled room (22 ± 2°C) with relative humidity (44-55%) under 12/12 h light and dark cycles for 1 week before and during the experiments. Animals were provided with a standard rodent pellet diet and clean drinking water ad libitum. Animals were divided into seven groups of six animals each:
-
Group I served as a sham for both prophylactic and curative studies and received raw water and free access to food for 16 days
-
Group II served as a normal control for both prophylactic and curative studies and received distilled water for 16 days orally and on the 16th day received olive oil (1 ml/kg body weight; i.p.)
-
Group III served as a toxic control for both prophylactic and curative studies and received distilled water for 16 days orally and on the 16th day received CCl4 (1 ml/kg body weight; 80% in olive oil)
-
Groups IV and V served as pre-treatment groups (prophylactic). They received CMFE at doses of 300 and 700 mg/kg, orally for 16 days respectively and on the 16th day received CCl4 (1 ml/kg body weight; 80% in olive oil), 2 h after administration of the last dose of extract
-
Groups VI and VII served as post-treatment groups (curative). They received distilled water orally for 16 days and on the 16th day they received CCl4 (1 ml/kg body weight; 80% in olive oil), followed by CMFE at doses of 300 mg/kg and 700 mg/kg (orally) respectively to Groups VI and VII at 2, 6, 12, 24 and 48 h after CCl4 intoxication.
Assessment of renal functions
All animals were sacrificed 50 h after CCl4 administration. Blood samples were collected from left ventricle. Serum was separated by centrifugation at 3000 rpm for 15 min and used for biochemical estimations and was used freshly for the assessment of kidney function tests. The urea, acid uric, creatinine and total protein levels were estimated by standard diagnostic test kits (Pars Azma, Iran).
Preparation of kidney homogenate
Renal tissues were homogenized in KCl (10 mM) phosphate buffer (1.15%) with EDTA: pH 7.4 and centrifuged at 12,000 rpm for 20 min. The supernatant was used for the measurement of malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx). Total protein contents were determined by the method of Lowry et al. (1951),[15] using bovine serum albumin as a standard.
Measurement of lipid peroxidation
Lipid peroxidation was measured by the TBA reaction method.[16] In brief, samples were mixed with TBA reagent consisting of 0.375% TBA and 15% TCA in 0.25-N hydrochloric acid (HCl). The reaction mixtures were placed in a boiling water bath for 30 min and centrifuged at 2500 rpm for 5 min. The absorbance of the supernatant was measured at 535 nm. MDA, a measure of lipid peroxidation, was calculated using an extinction coefficient of 1.56 × 105/M cm. The results were expressed as nmol/mg protein.
Determination of antioxidant enzymes
CAT activity was measured according to the method of Aebi (1984).[17] One unit of CAT was defined as the amount of enzyme required to decompose 1 μM of H2O2 in 2 min. The reaction was initiated by the addition of 1.0 ml of freshly prepared 20 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm for 1 min. The enzyme activity was expressed as U/mg protein.
The activity of SOD was measured according to the method of McCord et al. (1994).[18] For the determination of SOD activity, xanthine and xanthine oxidase were used to generate superoxide radicals reacting with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium chloride to form a red formazan dye. SOD activity was measured at 505 nm. Data were expressed as U/mg of protein.
GPx activity was determined by the method described by Paglia and Valentine (1967).[19] The reaction mixture consisted of 400 μl, 0.25 M potassium phosphate buffer (pH 7.0), 200 μl supernatant, 100 μl GSH (10 mM), 100 μl NADPH (2.5 mM) and 100 μl glutathione reductase (6 U/ml). Reaction was started by adding 100 μl hydrogen peroxide (12 mM) and absorbance measured at 366 nm at 1 min intervals for 5 min using a molar extinction coefficient of 6.22 × 103/M cm. Data were expressed as U/mg of protein.
Histopathology
For microscopic evaluation kidneys were fixed in a fixative (absolute alcohol 60%, formaldehyde 30% and glacial acetic acid 10%) and embedded in paraffin, sectioned at 4 μm, stained with hematoxylin/eosin and observed under a light microscope.
Animals rights and ethics
The study was cleared by the Institutional Animal Ethical Committee of Liver and Gastrointestinal Diseases Research Center (LGDRC) (No 91/232 – LGDRC).
Statistical analysis
All results are expressed as mean ± standard error of the mean one way analysis of variance followed by multiple comparison. Chi-square test with the Tukey post-hoc and independent samples T-test was used to compared different parameters. P < 0.05 was considered to be significant.
Results
Effect of CMFE on serum profile in rat
The effect of CCl4 administration on serum concentration of urea, acid uric, creatinine, protein and albumin are presented in Table 1. Administration of CCl4 to rats significantly (P < 0.05) increased the level of creatinine, urea and uric acid while the protein and albumin levels were reduced in comparison with the control group. Treatment of CMFE significantly (P < 0.05) reversed the changed levels of the above markers.

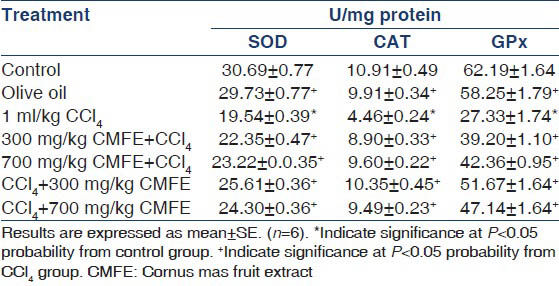
Effect of CMFE on renal antioxidant enzymes activity
Antioxidant enzymes activity in CCl4 group was found to be lower than in the normal group. The activity of these enzymes in treatment groups (pre and post) were significantly (P < 0.05) increased when compared with CCl4 group that shown in Table 2.
Effect of CMFE on renal contents of MDA
Effect of CCl4 and CMFE on renal MDA is shown in Figure 1. Administration of CCl4 to rats significantly (P < 0.05) induced lipid peroxidation as evidenced by the increased level of MDA. Treatment with CMFE protected against the CCl4 induced oxidative stress by reducing the lipid peroxidation.
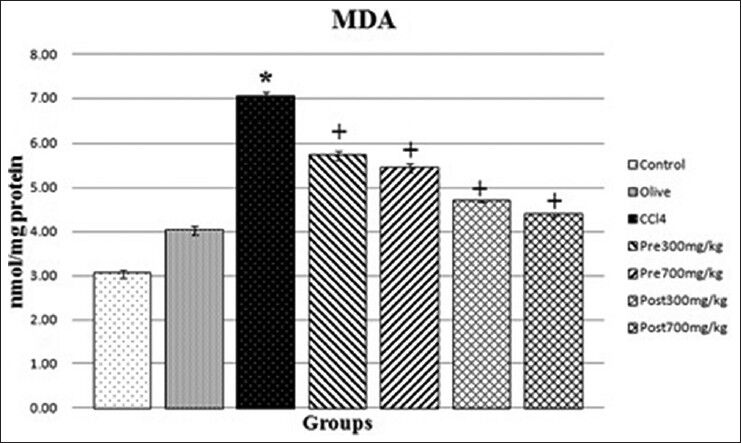
- Effect of CMFE on renal contents of MDA in rat. *Indicate significance at P < 0.05 probability from the control group. +Indicate significance at P < 0.05 probability from CCl4 group. CMFE, Cornus mas fruit extract; MDA, malondialdehyde
Renal histopathology
Administration of CCl4 caused glomerular and tubular injury. Treatment with CMFE ameliorated the changes to near normal histology [Figure 2].

- H and E stain: (a) Representative section from the control group. (b) CCl4 (1 ml/kg) group. (c) Olive oil (1 ml/kg) group. (d) CMFE (300 mg/kg) + CCl4 group. (e) CMFE (700 mg/kg) + CCl4 group. (f) CCl4 + CMFE (300 mg/kg) group. (g) CCl4 + CMFE (700 mg/kg) group. CMFE, Cornus mas fruit extract; CCl4, carbon tetrachloride; DBC, damaged Bowman capsule; TD, tubular degeneration; LBB, loss of border brush; PC, proximal tubule; DT, distal tubule; NGB, normal glomerulus and Bowman capsule; BB, border brush; FA, foamy appearance
Discussion
The present study demonstrated the protective potential of CMFE on CCl4 induced nephrotoxicity. CCl4 has been used in rat experimental models to investigate the oxidative stress induced in various organs. To the best of our knowledge, this is the first study to evaluate these effects of CMFE in an attempt to prevent kidney damage from CCl4.
The mechanism of CCl4 hepatotoxicity is well documented in the rat model.[142021] CCl4 intoxication generates free radicals that trigger a cascade of events resulting in organ toxicity in rats. It is well-known that the kidneys play a pivotal role in the regulation of various chemicals. CCl4, a nephrotoxin, was used for the purpose of inducing renal damage in this study since it has previously been shown to exert its toxic effects on the kidney.[2223] According to previous reports, CCl4-induced toxicity is due to the conversion of CCl4 to CCl3· and CCl3O2· by the liver cytochrome P450 enzyme. These highly reactive free radicals cause cell damage.
Elevations in the serum concentrations of urea and creatinine as seen here are indicative of renal injury, which was accompanied by histological changes.[32324] In addition, reduced level of serum albumin and protein in CCl4-treated rats might have resulted from leakage in glomeruli and tubules. Similar results have been shown by Khan et al. The present study revealed that the treatment of CMFE to CCl4-administrated rats ameliorated the toxic affect of CCl4. Results obtained in this study are in agreement with earlier findings.[25]
The lipid peroxidation is an autocatalytic process and common consequence of cell death. It causes tissue damage during inflammation, cancer and aging.[26] Lipid peroxidation is reported to be a major causes of CCl4-induced nephrotoxicity, mediated by the production of free radical derivatives of CCl4. The renal MDA content, which is one of the end products of lipid peroxidation in the renal tissue, is used as an important indicator of CCl4-induced oxidative stress. In the present study, administration of CCl4 resulted in significant elevation in MDA concentration [Figure 1] indicating elevation of lipid peroxidation along with histopathological injury [Figure 2]. Interestingly, treatment by CMFE markedly the MDA concentration.
Although there are numerous studies demonstrating that CCl4 leads to increase in MDA levels in various tissues,[2728] a limited number of studies have investigated the effects of CMFE. In addition, no published data has ever demonstrated the influence of CMFE on CCl4-induced elevation in renal lipid peroxide levels.
It has been suggested that a decrease in the activities of primary antioxidant; CAT, SOD and GPx may be due accumulation of ROS. An observation that strengthens this hypothesis is that SOD activity can be inhibited by hydrogen peroxide treatment.[29] The inhibition of antioxidant system may lead to accumulation of H2O2 or products of its decomposition may also be aided by a decrease in CAT, SOD and GPx activities.[230] Measurement of these antioxidant enzymes is an appropriate indirect way to assess the pro-oxidant antioxidant status in tissues.[3031] The level of antioxidant enzymes such as SOD, CAT and GPx decreased in CCl4-treated group, and improved by treatment with CMFE. Results obtained in this study suggest the protective effects of CMFE against the CCl4-induced nephrotoxicity, could be attributed to its high level of phenol[32] and other antioxidants.[3334353637] These compounds could scavenge the free radicals of CCl4 generated through P450 enzyme system thereby diminished the oxidative injuries.
In this study, the kidneys of CCl4-treated rats have shown severe morphological abnormalities in the glomerular and tubular compartments. These changes were not observed in the groups treated with CMFE, that suggesting the protective effects of CMFE in attenuating CCl4-induced morphological changes.
Conclusion
The present study suggests the antioxidant potential of CMFE against the toxic effects of CCl4 in the kidney of rats. Research is needed about each of these components against CCl4 induced nephrotoxicity.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Carbon tetrachloride-induced nephrotoxicity in rats: Protective role of Digera muricata. J Ethnopharmacol. 2009;122:91-9.
- [Google Scholar]
- Evaluation of Launaea procumbens use in renal disorders: A rat model. J Ethnopharmacol. 2010;128:452-61.
- [Google Scholar]
- Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;97:273-80.
- [Google Scholar]
- Toxicological profile for carbon tetrachloride. Agency for Toxic Substances and Disease Registry 2005
- [Google Scholar]
- Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis. 2010;17:254-64.
- [Google Scholar]
- Comparative metabolism of carbon tetrachloride in rats, mice, and hamsters using gas uptake and PBPK modeling. J Toxicol Environ Health A. 2000;60:531-48.
- [Google Scholar]
- Oxidative damage to the lipids and proteins pf the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin Chim Acta. 1999;289:177-9.
- [Google Scholar]
- Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res. 2010;10:153-164.
- [Google Scholar]
- Protective effects of total flavonoids of Bidens bipinnata L. against carbon tetrachloride-induced liver fibrosis in rats. J Pharm Pharmacol. 2008;60:1393-402.
- [Google Scholar]
- Protective effect of Cichorium glandulosum root extract on carbon tetrachloride-induced and galactosamine-induced hepatotoxicity in mice. Food Chem Toxicol. 2009;47:2022-30.
- [Google Scholar]
- Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207-13.
- [Google Scholar]
- Antioxidant properties of some plants growing wild in Turkey. Grasas y Aceites. 2009;60:147-54.
- [Google Scholar]
- Does oxidative protein damage play a role in the pathogenesis of carbon tetrachloride-induced liver injury in the rat? Biochim Biophys Acta. 1997;1362:169-76.
- [Google Scholar]
- The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol Carcinog. 1998;23:175-84.
- [Google Scholar]
- Mutant mice, Cu, Zn superoxide dismutase, and motor neuron degeneration. Science. 1994;266:1586-7.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-69.
- [Google Scholar]
- Protective role of propolis against alcohol carbon tetrachloride-induced hepatotoxicity in rats. Indian J Pharmacol. 1997;29:76.
- [Google Scholar]
- Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride-induced acute hepatotoxicity in the rat. J Ethnopharmacol. 2002;81:145-54.
- [Google Scholar]
- Protective effect of interferon-alpha on carbon tetrachloride-induced nephrotoxicity. J Nephrol. 2003;16:81-4.
- [Google Scholar]
- Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology. 2003;62:353-6.
- [Google Scholar]
- Furosemide enhancement of experimental gentamicin nephrotoxicity: Comparison of functional and morphological changes with activities of urinary enzymes. J Infect Dis. 1979;140:342-52.
- [Google Scholar]
- Hsian-tsao (Mesona procumbens Heml.) prevents against rat liver fibrosis induced by CCl (4) via inhibition of hepatic stellate cells activation. Food Chem Toxicol. 2008;46:3707-13.
- [Google Scholar]
- Reversal of acetaminophen induced subchronic hepatorenal injury by propolis extract in rats. Environ Toxicol Pharmacol. 2009;27:17-25.
- [Google Scholar]
- Melatonin counteracts lipid peroxidation induced by carbon tetrachloride but does not restore glucose-6 phosphatase activity. J Pineal Res. 1995;19:1-6.
- [Google Scholar]
- Investigation of antioxidant effect of melatonin against carbon tetrachloride toxicity in various tissues. Biomed Res. 1999;10:141-5.
- [Google Scholar]
- Effect of acute vs chronic H2O2-induced oxidative stress on antioxidant enzyme activities. Free Radic Res. 2009;43:340-7.
- [Google Scholar]
- Reactive oxygen species in pathology with special reference to the skin Oxidative Stress in Dermatology. New York: Marcek Dekker Inc; 1993.
- [Google Scholar]
- Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact. 2009;179:118-24.
- [Google Scholar]
- Nutritional quality of some wild leafy vegetables in South Africa. Int J Food Sci Nutr. 2009;60:424-31.
- [Google Scholar]
- Sesquiterpene lactone glycosides and ionone derivative glycosides from Sonchus asper. Phytochemistry. 1989;28:3399-402.
- [Google Scholar]
- A chemotaxonomic survey of Sonchus subgenus Sonchus. Biochem Syst Ecol. 1993;21:617-20.
- [Google Scholar]
- A chematoxonomic review of the subtribe crepidinase based on its phenol constituents. Biochem Syst Ecol. 1994;22:297-305.
- [Google Scholar]
- Nutritional composition of Sonchus species (S asper L., S oleraceus L and S tenerrimus L) J Sci Food Agric. 1998;76:628-32.
- [Google Scholar]
- Sesquiterpene lactone glucosides from Sonchus asper. Phytochemistry. 2000;53:473-7.
- [Google Scholar]







