Translate this page into:
Adefovir nephrotoxicity in a renal allograft recipient
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Adefovir dipivoxil, an oral prodrug of adefovir, is used in the treatment of lamivudine-resistant hepatitis B virus (HBV) infection. Nephrotoxicity manifesting as proximal renal tubular dysfunction and acute tubular necrosis (ATN) were commonly reported in the past, when higher doses were used for the treatment of human immunodeficiency virus infection. However, nephrotoxicity is rare at lower doses that are currently recommended for the treatment of HBV infection. A 31-year-old female was detected to be hepatitis B surface antigen positive months after a kidney transplant. The patient was initiated on lamivudine, but developed resistance after 1 year of treatment, at which time low-dose adefovir was added. The patient developed renal allograft dysfunction after 10 months of starting adefovir. Serum creatinine increased from 1.1 mg/dl to 1.9 mg/dl, along with progressively increasing sub-nephrotic proteinuria. Renal allograft biopsy revealed features of ATN. After discontinuation of adefovir, proteinuria resolved and renal dysfunction improved slowly over the next 2 years. Adefovir-induced nephrotoxicity, although uncommon at lower doses, needs to be considered in the differential diagnosis of renal dysfunction and sub-nephrotic proteinuria occurring in patients receiving adefovir for prolonged periods.
Keywords
Adefovir
hepatitis B
lamivudine-resistant
nephrotoxicity
renal
transplant.
Introduction
Effective treatment of hepatitis B virus (HBV) in renal transplant recipients is required to prevent active viral replication and progression of liver damage.[12] Lamivudine has traditionally been considered to be the drug of choice for post-transplant HBV infection due to its efficacy and safety.[3] However, there is an increasing incidence of lamivudine resistance due to selection of tyrosine-methionine-aspartate-aspartate (YMDD) mutants after long term therapy with lamivudine.[4] Adefovir dipivoxil, an oral prodrug of adefovir, is one of the agents effective against lamivudine-resistant HBV. Nephrotoxicity manifesting as proximal renal tubular dysfunction and acute tubular necrosis (ATN) were commonly reported, when higher doses (60–120 mg/day) of adefovir were used for the treatment of human immunodeficiency virus (HIV) infection in the past.[5] However, nephrotoxicity is quite rare at lower doses (10 mg/day) currently recommended for the treatment of HBV infection. In this report, we describe the occurrence of adefovir-induced ATN in a renal allograft recipient who was treated with adefovir 10 mg daily for lamivudine-resistant HBV infection.
Case Report
A 31-year-old female with end-stage renal disease due to immunoglobulin A nephropathy underwent living-related kidney transplantation in May 2006, with mother as voluntary kidney donor. The human leukocyte antigen was haplomatch and pre-transplant complement-dependent cytotoxicity cross-match was negative. Her pre-transplant HIV enzyme-linked immunosorbent assay, hepatitis B surface antigen (HbsAg) and anti-hepatitis C virus serology was negative. She did not receive induction immunosuppression. There were no intraoperative or postoperative complications. She did not require blood transfusion during surgery. She was on prednisolone, cyclosporine and azathioprine for maintenance immunosuppression and reached a nadir serum creatinine of 0.7 mg/dl on the 5th postoperative day. She was detected to have BK viremia 1 month after the transplantation without graft dysfunction and was initiated on leflunomide 20 mg once daily. Her cyclosporine dose was gradually reduced from an initial dose of 150 mg twice daily in 2006 to 75 mg twice a day in 2007 and 25 mg twice daily in 2008. The same dose of cyclosporine was continued henceforth along with 7.5 mg of prednisolone, 100 mg azathioprine and 20 mg leflunomide. She had a stable graft function with serum creatinine of 1.1 mg/dl on this immunosuppression.
Fifteen months after the transplantation, she was incidentally detected to have deranged liver enzymes with aspartate aminotransferase and alanine aminotransferase levels of 60 IU/ml and 87 IU/ml, respectively. On further evaluation, she was detected to be HbsAg positive with HBV deoxyribonucleic acid titers of 2 × 108 IU/ml. She was initiated on lamivudine 100 mg once daily. The HBV DNA titers decreased to 466 IU/ml after 6 months. But on repeat evaluation a year later, HBV titers had rebounded to 1 × 107 IU/ml despite good compliance. In view of lamivudine resistance, she was advised to change to entecavir. However due to financial constraints, adefovir 10 mg once daily was added to lamivudine in August 2008. Her serum creatinine then was 1.1 mg/dl, with normal 24-h urine protein excretion. Her other medications included calcium carbonate, calcitriol, amlodipine, atorvastatin and leflunomide, apart from maintenance triple immunosuppression.
After the addition of adefovir, her HBV DNA titers decreased to 79 IU/ml. However, there was slow deterioration of renal allograft function with serum creatinine increasing to 1.5 mg/dl after 10 months of adefovir and then to 1.9 mg/dl after 26 months of adefovir therapy. Simultaneously, there was progressive increase in 24-h urine protein excretion to 847 mg/dl. Her serum phosphorus was 3.1 mg/dl (normal range: 2.5–4.6 mg/dl) and serum bicarbonate was 24 meq/L. The urine pH was 7.0 with no glycosuria on dipstick analysis. BK virus polymerase chain reaction in blood was negative. No changes in immunosuppression were made during this period.
A renal biopsy was performed, which revealed a core of renal tissue with 12 glomeruli, of which 3 were obsolescent. The remaining glomeruli displayed mild increase in mesangial cellularity. There were focal areas of ATN with regenerative changes of tubular lining epithelium, along with interstitial edema and mild interstitial infiltrates of mononuclear cells [Figure 1]. There was no evidence of rejection. Immunofluorescence staining did not reveal any immune deposits. C4d staining of peritubular capillaries was negative.
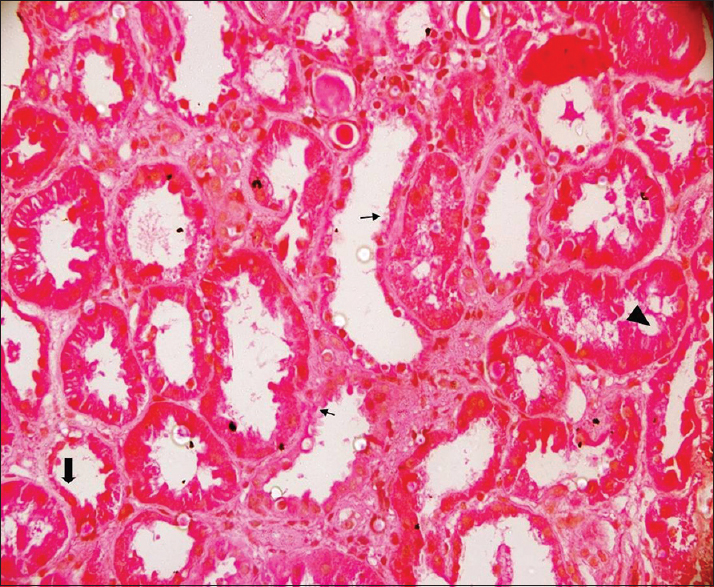
- Renal allograft biopsy revealing features of acute tubular necrosis. Biopsy showing tubular cell swelling (arrow head), cell necrosis with denudation of the basement membrane (thin arrows), and thinning of the brush-border (thick arrow)
The possibility of adefovir-induced ATN was considered and adefovir was replaced with entecavir. At the subsequent visit 6 months later, proteinuria had resolved completely to 89 mg/day, while serum creatinine improved to 1.4 mg/dl. Over the next 18 months, renal function continued to improve with serum creatinine reaching the baseline of 1.2 mg/dl [Figure 2]. However, since the patient did not achieve adequate viral suppression with entecavir at doses recommended in the setting of lamivudine resistance and due to lack of safer alternatives, tenofovir disoproxil fumarate was added to entecavir, with close monitoring of serum creatinine and tubular function. At the last follow-up, the patient is having stable renal allograft function with negative HBV DNA titers.
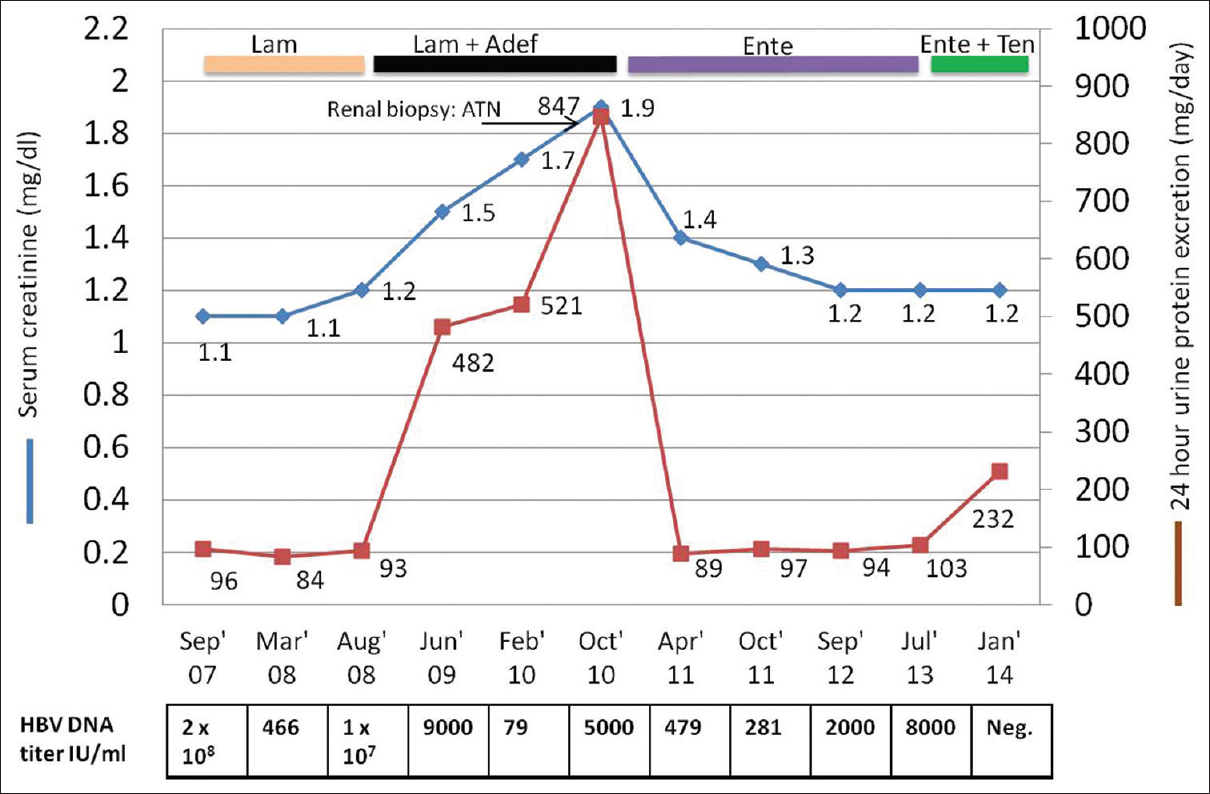
- Timeline of renal dysfunction and urinary protein excretion in relation to adefovir initiation. (Adef: Adefovir; ATN: Acute tubular necrosis; Ente: Entecavir; Lam: Lamivudine; Ten: Tenofovir)
Discussion
Adefovir dipivoxil is an oral prodrug of adefovir that is effective against lamivudine-resistant HBV mutants. Adefovir was originally developed as an antiretroviral agent for HIV at a dosage of 60–120 mg daily, but was not preferred later due to high incidence of nephrotoxicity, occurring in 22–50% of patients.[5] Subsequently it was found that adefovir was effective against HBV in much lower dosages and was approved for treatment of the same in 2002 at a dosage of 10 mg daily. The active intracellular metabolite, adefovir diphosphate causes inhibition of both reverse transcriptase and HBV DNA polymerase and is incorporated into DNA causing chain termination, accounting for its therapeutic effects.
The drug gets concentrated within the renal proximal tubular cells due to active uptake from the blood by the human organic anion transporter-1.[6] It is then secreted into the urine by multi-drug resistant protein located at the apical side of proximal tubular cells. Adefovir acts as a mitochondrial toxin due to its ability to inhibit the human mitochondrial DNA polymerase gamma.[7] This can lead to impaired mitochondrial replication with mitochondrial loss and dysfunction, with resultant impairment of oxidative phosphorylation and cellular damage.
In the initial 1-year registration trials for HBV infection, the frequency of grade I nephrotoxicity (defined as serum creatinine 0.5 mg/dl above baseline values) was similar in adefovir-treated and placebo-treated patients (0% vs. 0%).[89] However, long-term studies reported that adefovir-induced nephrotoxicity occurred in a small proportion of patients even at lower dosages. For example, in a cohort of 125 patients with chronic hepatitis B e antigen (HBeAg)-negative hepatitis B treated with adefovir for 5 years, the frequency of serum creatinine elevations that were 0.5 mg/dl above baseline was 3%.[10] Similarly, 8% of the 65 patients who were HBeAg-positive and receiving adefovir for 5 years had reversible creatinine elevations, 5% had albuminuria, and 3% developed hypophosphatemia.[11]
From these observations, it is clear that the nephrotoxicity of adefovir is both dose- and time-dependent. The typical clinical pattern of adefovir nephrotoxicity is characterized by slow rises in serum creatinine and decreases in serum phosphate levels, occurring 4–12 months after starting therapy. This can be associated with Fanconi-like renal tubular acidosis. The nephrotoxicity is usually reversible if therapy is stopped promptly.
In our patient, the pattern of nephrotoxicity was similar, as characterized by a gradual and progressive rise in serum creatinine along with tubular-range proteinuria over a period of 2 years. The renal biopsy revealed characteristic features of ATN such as thinning of the brush-border, tubular cell swelling and cell necrosis with denudation of the basement membrane. The other findings noted such as mild increase in mesangial cellularity, interstitial edema and mild interstitial infiltrates were not significant enough to suggest an alternative diagnosis. There was no evidence of tubulitis or peritubular capillaritis to merit a diagnosis of graft rejection. A review of the patient's medications did not reveal any confounding agent. The serum level of cyclosporine was < 25 ng/ml, thus making calcineurin nephrotoxicity highly unlikely.
On withdrawal of adefovir, there was prompt resolution of proteinuria and improvement in serum creatinine to 1.4 mg/dl. However, the serum creatinine reached baseline only after a period of around 2 years. Delay in recovery of renal function following withdrawal of adefovir has been described previously.[512] For example, in a randomized, double-blind, placebo-controlled multicenter trial with 12 months' follow-up among HIV-infected patients, 253 patients were assigned to adefovir 120 mg once daily.[5] Among 17.2% patients who developed adefovir nephrotoxicity, the median time to resolution of proximal renal tubular dysfunction was 15 weeks and in 16% of patients, the renal function did not recover completely even at 41 weeks after onset of nephrotoxicity.[5] Considering that the above study was conducted in patients with native kidneys, further delay in recovery of ATN can be expected in renal allograft recipients. For instance, in a report of adefovir-induced ATN in a renal allograft recipient, the renal function continued to worsen after discontinuation of adefovir, even though other markers of proximal tubular dysfunction resolved at 4 months after discontinuation.[12] Delay in recovery of ATN following delayed graft function has also been reported in renal allograft recipients.[13]
Our patient did not have features of proximal renal tubular acidosis or hypophosphatemia, illustrating that all features of nephrotoxicity need not manifest in a particular patient. Thus, a high index of suspicion is required to diagnose adefovir nephrotoxicity.
The occurrence of adefovir nephrotoxicity in renal allograft recipients has been reported rarely. In a series of 11 renal transplant recipients who received adefovir for lamivudine-resistant chronic HBV infection, at 2 years after starting adefovir, there was a significant increase in serum creatinine from 125 (±35) to 141 (±32) μmol/l, (P = 0.02) and a significant increase in 24-hour proteinuria.[14] In another case series involving 14 renal transplant recipients who were on adefovir, four patients (29%) developed renal dysfunction due to presumed adefovir nephrotoxicity.[15]
Conclusion
Adefovir-induced nephrotoxicity, although uncommon at lower doses, needs to be considered in the differential diagnosis of renal dysfunction and sub-nephrotic proteinuria occurring in patients receiving adefovir for prolonged periods.
Source of Support: Nil
Conflict of Interest: None declared.
References
- HBsAg seropositive status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant. 2005;5:2913-21.
- [Google Scholar]
- Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257-63.
- [Google Scholar]
- Lamivudine for the treatment of hepatitis B virus-related liver disease after renal transplantation: Meta-analysis of clinical trials. Transplantation. 2004;77:859-64.
- [Google Scholar]
- Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711-6.
- [Google Scholar]
- The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: A randomized, placebo-controlled trial. AIDS. 2001;15:1695-700.
- [Google Scholar]
- Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11:383-93.
- [Google Scholar]
- Adefovir nephrotoxicity: Possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32:734-40.
- [Google Scholar]
- Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-16.
- [Google Scholar]
- Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-7.
- [Google Scholar]
- Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-51.
- [Google Scholar]
- Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750-8.
- [Google Scholar]
- Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome in a renal transplant patient. AIDS. 2009;23:544-5.
- [Google Scholar]
- Post transplant acute tubular necrosis - How long you can wait. A case report? Saudi J Kidney Dis Transpl. 2002;13:50-4.
- [Google Scholar]
- Renal side effects of adefovir in hepatitis B virus-(HBV) positive kidney allograft recipients. Clin Nephrol. 2009;71:36-42.
- [Google Scholar]
- Safety and efficacy of adefovir therapy for lamivudine-resistant hepatitis B virus infection in renal transplant recipients. J Formos Med Assoc. 2012;111:439-44.
- [Google Scholar]







