Translate this page into:
Chronic active antibody mediated rejection associated with human leukocyte antigen-C*07 antibodies
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
The detection of donor specific antibody (DSA) is critical to the diagnosis of antibody mediated rejection (AMR)[12] DSAs against the human leukocyte antigen (HLA) antigens are found in more than 90% of the AMR cases, while the minority can have minor-HLA or non-HLA antibodies.[3] The diagnosis of AMR is significant for the management and long-term outcome of the transplant recipient. Most of the AMRs are known to be associated with DSAs against HLA-A, HLA-B, and HLA-DRB1.
A 25-year-old male received a transplant with a kidney from his mother (3/6 HLA match) 3 years ago. The HLA typing was performed using PCR-SSP method (Invitrogen, Life Technologies, NY, USA). The HLA type of the donor was HLA-A*03, A*33, HLA-B*51, B*55 and HLA-DRB1*04, DRB1*04, while that of the patient (who was a haplomatch) was HLA-A*02, A*33, HLA-B*55, B*55 and HLA-DRB1*01, DRB1*04. The pretransplant complement-dependent cytotoxicity crossmatch was negative (cell death <20%). The flow cytometric crossmatch, performed on BD FACSCalibur® flow cytometer (BD Biosciences, CA, USA), was also negative for both B- and T-cells (Cut-off: Channel shift of 50 and 80 for T- and B-cells, respectively). Immunosuppression consisted of tacrolimus, mycophenolate mofetil, and steroids (without induction). The immediate graft function was good and he was doing well on follow-up with a baseline creatinine of 1.5 mg/dl. He presented to us with rapid worsening of the kidney function with serum creatinine of 3.5 mg/dl. He denied any compliance issues with immunosuppressive medications, and the tacrolimus level 1-day prior to the admission was 7 ng/ml.
In view of graft dysfunction, a graft biopsy was performed which showed extensive neutrophilic glomerulitis (g3, Figure 1a) and the duplication of basement membrane (cg2). Tubulointerstitial compartment showed acute tubular injury with moderate patchy interstitial infiltrate occupying 20–25% of the sampled cortex (i1). The interstitial infiltrate showed the prominence of plasma cells. Peritubular capillaritis was marked and showed neutrophilic dominance (ptc3). Immunofluorescence core revealed negativity for IgG, IgM, C1q, and kappa and lambda with IgA being traces to 1+granular in mesangium. C4d was diffusely positive in peritubular capillaries (C4d3, Figure 1b) along with strong staining along glomerular capillary loops. Banff score was g3 cg2 mm0 t1 ct0 i1 ci0 v0 cv0 ah0 ptc3. C4d3. The microscopy and immunofluorescence studies were suggestive of chronic active AMR. There was no significant tubulointerstitial fibrosis.
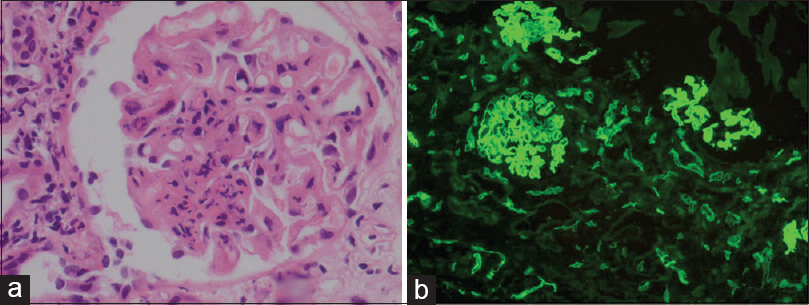
- (a) Renal biopsy showing marked neutrophilic glomerulitis (H and E, ×400). (b) C4d positivity in peritubular capillaries, IF ×100
DSA testing was performed by Luminex® Single Antigen Bead based assay using Lifecodes® LSA Class I and Class II kits (Immucor, Inc, GA, USA). Relevant quality control parameters were within normal limits. It revealed the presence of antibodies against HLA-C*07, HLA-C*12, and HLA-C*17 [Figure 2]. There were no antibodies against any of the HLA-A, HLA-B antigens, or the class II antigens included in the panel (i.e., DRB1, DPB1, or DQB1). The MFI of these antibodies ranged from 1346 to 1955. The MICA (MHC Class I polypeptide-related sequence A) antibody testing of the patient serum was also performed, using Lifecodes LSA-MIC kit (Immucor, Inc, GA, USA) and it did not reveal any antibody.
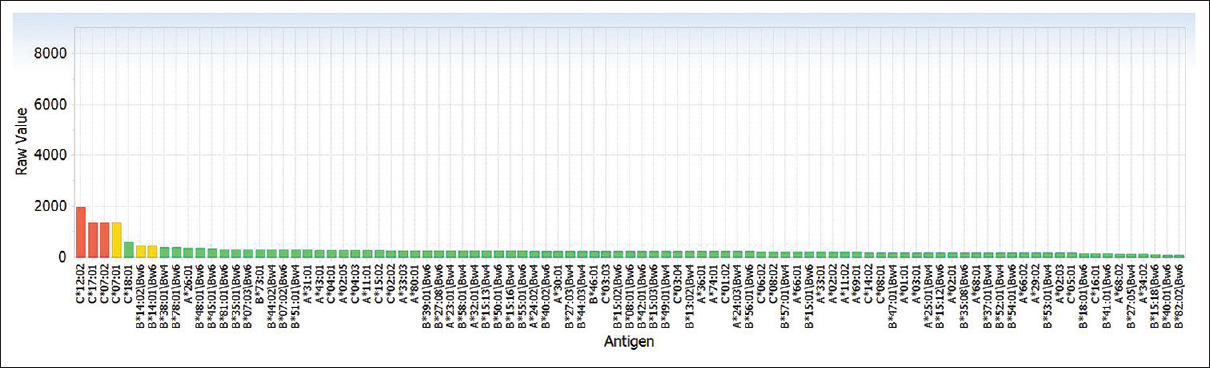
- Graph depicting positivity for human leukocyte antigen-C*07, C*12, and C*17 on Luminex single antigen bead assay
We routinely perform the HLA typing of the recipient and the donor only for HLA-A, HLA-B, and HLA-DRB1 loci in our center. The patient and the donor were haploidentical. However, the typing for HLA-C locus was not available, and therefore, it was not possible to decide if the HLA class I antibodies against HLA-C*07, *12, and *17 identified were donor specific or not. HLA-C locus typing was hence performed on the preserved DNA sample of the donor and recipient. HLA-C of the donor was C*03 and C*07, while that of the patient was C*03 and C*03.
Hence, the chronic AMR in the index case could be ascribed to the presence of anti-HLA-C*07 antibodies, which were donor specific. After the diagnosis, the patient was treated with 3 sessions of plasma exchange and 3 mg/kg anti-thymocyte globulin (ATG). We had planned to give 5 sessions of plasmapheresis and up to 6 mg/Kg ATG but this could not be achieved due to financial constraints. The clinical response was sub-optimal and on the latest follow-up, the serum creatinine was stable at 3.2 mg/dl.
The presence and identification of donor-specific HLA antibodies are critical for the diagnosis of AMR and its management. Most AMRs are known and considered to be due to antibodies against HLA-A, B, and DRB1. In India, these three loci are the ones that are typed in most of the centers in the pretransplant setting and HLA-C testing is not routinely performed. HLA-C antibodies were not considered to be too significant until its role was documented in 2001.[4] Frohn et al. found a role of HLA-C mismatch if it was accompanied by HLA-B mismatch.[5] A mismatch in the HLA-DR locus is traditionally considered to have the greatest influence on the probability of subsequent rejection followed by HLA-B locus mismatches. Mismatches at HLA-C locus has been documented to be important for compatibility in hematopoietic stem cell transplantation, and hence, its immunogenic role cannot be discounted.[6] It has also been observed that HLA-C disparity results in significant graft failure.[7] Similar views have been echoed by Duquesnoy RJ, (2001), where the authors documented the importance of HLA-C mismatch in humoral sensitization and that HLA-C epitopes can induce specific antibodies as they found the donor specific HLA-C antibody in the serum of 26 out of 45 HLA-C mismatched patients.[8] A similar finding was reported by Bachelet, et al. However, they could also elucidate the DSA against DPB1 in addition to HLA-C, which was not observed in the index case.[9]
This case highlights an important message, in particular, to the parts of the world where HLA-C typing is not routinely performed. This is either due to limited resources or due to the fact that the importance of HLA-C in clinical transplantation has only been recently recognized and therefore not well-known to the transplant community. Same would hold true for DP and DQ antigens which are not typed routinely. Therefore, we suggest that, where possible HLA typing should include these loci. In cases where this has not been done, and if an AMR develops subsequently and DSA is detected, additional HLA typing of the donor should be done to clarify- if these antibodies are donor specific. This will have relevance for both immediate and long-term management of the patient as well as to identify subsequent mismatch if the patient requires a second transplant
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779-87.
- [Google Scholar]
- Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272-83.
- [Google Scholar]
- Identification of the nonclassical HLA molecules, mica, as targets for humoral immunity associated with irreversible rejection of kidney allografts. Transplantation. 2002;74:268-77.
- [Google Scholar]
- Acute antibody-mediated renal allograft rejection associated with HLA-Cw17 antibody. Clin Kidney J. 2012;5:254-6.
- [Google Scholar]
- Acute antibody-mediated renal allograft rejection associated with HLA-Cw17 antibody. Nephrol Dial Transplant. 2001;16:355-60.
- [Google Scholar]
- A correlation between HLA-C matching and donor antirecipient CTL precursor frequency in bone marrow transplantation. Transplantation. 1996;61:1420-3.
- [Google Scholar]
- Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997;89:1818-23.
- [Google Scholar]
- Detection of antibodies against HLA-C epitopes in patients with rejected kidney transplants. Transpl Immunol. 2011;24:164-71.
- [Google Scholar]
- Anti-Cw donor-specific alloantibodies can lead to positive flow cytometry crossmatch and irreversible acute antibody-mediated rejection. Am J Transplant. 2011;11:1543-4.
- [Google Scholar]






