Translate this page into:
Nephroprotective effect of estrogen and progesterone combination on cisplatin-induced nephrotoxicity in ovariectomized female rats
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Recently, we reported that estrogen (Es) has no beneficial effect on cisplatin (CP)-induced nephrotoxicity, but the role of progesterone (Pr) and the combination of Es and Pr are not yet well-defined. In this study, we investigated the protective role of Pr, and co-administration of Es/Pr on CP-induced nephrotoxicity. Eighty-six ovariectomized female Wistar rats were divided into 13 groups, and the experiments were performed in two phases. In Phase I, Groups 1–4 received 2, 5, 10, and 25 mg/kg, IM Pr dissolved in sesame oil every 5 days for four doses. Groups 5–8 had the same treatment regimen as Groups 1–4, but after the third injection the animals also received continuous dose of CP (2.5 mg/kg/day, i.p.) for 8 days. Group 9, as the positive control group, received sesame oil instead of Pr plus CP. Group 10, as the negative control group, received sesame oil instead of Pr. After the most effective dose of Pr was determined in Phase I, Groups 11–13 in Phase II received 10 mg/kg Pr plus either 0.25, 0.5, or 1 mg/kg, IM estradiol valerate every 5 days for four doses. After the third injection, they also received a continuous dose of CP for 8 days. The levels of blood urea nitrogen (BUN) and creatinine (Cr), kidney tissue damage score (KTDS), and kidney weight (KW) increased and body weight (BW) decreased in the positive control group (P < 0.05). Administration of Pr (10 mg/kg) plus CP decreased KTDS and BW loss and KW. Co-administration of ES/Pr at specific doses improved Cr, BUN, and KTDS; and resulted in reduced CP-induced nephrotoxicity. The results obtained suggest that the beneficial effect of Pr on CP-induced nephrotoxicity is dose-dependent. In addition, combination of Es/Pr with a specific dose decreased CP-induced nephrotoxicity.
Keywords
Cisplatin
estrogen
nephrotoxicity
progesterone
rats
Introduction
Cisplatin (CP) is used for the treatment of solid tumors including those of breast, head and neck, lung, testis, and ovary.[1] The most common side-effect of CP is nephrotoxicity.[123] Since CP increases the levels of reactive oxygen species (ROS),[1] several agents such as vitamins C and E, losartan (angiotensin II receptor blocker), erythropoietin, L-Arginine, N-acetylcysteine have been suggested as nephroprotective agents against CP-induced nephrotoxicity.[345678910] CP-induced nephrotoxicity is shown to be gender-dependent, with a male preponderance.[11] Considering the sexual dimorphism pattern of kidney diseases, in which men are more frequently experience renal dysfunction, male and female sex hormones possibly play a role.[1213] Experimental studies have shown an increase in renal dysfunction by androgens.[14] We have previously demonstrated that estrogen (Es) in pharmacologic doses exacerbate CP-induced nephrotoxicity, and the low pharmacologic dose does not have a beneficial effect.[15] The effect of fennel essential oil on CP-induced nephrotoxicity in estradiol-treated rats was tested and showed that fennel essential oil was not nephroprotective.[16] Progesterone (Pr) suppresses the production of pro-inflammatory cytokines and chemokines in monocytes.[17] Moreover, it has been shown that Pr receptor plays a major anti-inflammatory role in human myometrial cells.[18] It was demonstrated that Pr alone and in combination with Es arrest kidney damage in rats with Doca-Salt hypertension while this is not observed by the administration of Es alone.[19] This study was designed to find the protective role of Pr alone and combination of Es and Pr in CP-induced nephrotoxicity in ovariectomized rats.
Materials and Methods
Eighty-six adult female Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) (178.4 ± 1.4 g) were used. The animals were kept under standard conditions and were fed with rat chow and water. All experiments were approved by the Isfahan University of Medical Sciences Ethics Committee.
Experimental design
This study was run in two phases. In Phase I, we tried to find the most effective dose of Pr to reduce CP-induced nephrotoxicity. In the second phase, the selected dose of Pr was accompanied with three different doses of estradiol to determine the role of the combination of Es and ES/Pr on CP-induced nephrotoxicity.
The rats were ovariectomized as described previously.[2] After 5 days of recovery, the rats were randomly divided into 13 experimental groups and received one of the following treatments. In Phase I, Groups 1–4 received 2, 5, 10, and 25 mg/kg, IM Pr in sesame oil every 5 day for four doses. These groups were called Pr 2, Pr 5, Pr 10, and Pr 25 groups, respectively. Groups 5–8 (called Pr 2+CP, Pr 5+CP, Pr 10+CP, and Pr 25+CP groups, respectively) were considered as the case-study groups and respectively received 2, 5, 10, and 25 mg/kg, IM Pr in sesame oil every 5 day for four doses, and after the third injection the animals received continuous dose of CP (2.5 mg/kg, i.p.) for 8 days. Group 9 (called CP group) as the positive control group underwent treatment the same as Groups 5–8 but received sesame oil instead of Pr. Group 10 (called OV group), as the negative control group, received sesame oil alone instead of Pr and saline instead of CP. After determination of the most effective dose of Pr (10 mg/kg), Groups 11–13 (called ES 0.25 + Pr 10 + CP, ES 0.5 + Pr 10 + CP, and ES 1 + Pr 10 + CP groups, respectively) as the case-study groups received 10 mg/kg Pr plus 0.25, 0.5, or 1 mg/kg IM estradiol valerate in sesame oil every 5 day for four doses and after the third injection, they received continuous dose of CP for 8 days. The weight of animals was recorded daily. After the last CP injection, the rats were anesthetized with chloral hydrate (450 mg/kg), blood samples were obtained by heart puncture, and the rats were sacrificed. Kidneys were removed and weighted immediately and left kidneys were prepared for histopathological procedures. The uterus was also removed and weighted. Diagram 1 demonstrates the experimental design of this study.[INLINE:1]

- The experimental design of the study. Phase I: Groups 5–8 received 2, 5, 10, and 25 mg/kg, progesterone (Pr) every 5 days during the study (total of 4 doses), while after the third injection of Pr the animals also received continuous dose of cisplatin (CP, 2.5 mg/kg/day) for 8 days. Groups 1–4 had the same treatment regimen as Groups 5–8, but saline instead of CP. Group 9, as the positive control group, received sesame oil instead of Pr plus CP. Group 10, as the negative control group, received sesame oil instead of Pr plus saline instead of CP. Phase II: Groups 11–13 in phase II received 10 mg/kg Pr plus either 0.25, 0.5, or 1 mg/kg, im estradiol valerate (Es) every 5 days (total of 4 doses) while after the third injection, they also received continuous dose of CP for 8 days (e)
Drugs
Estrogen was purchased from Aburaihan Co. (Tehran, Iran), CP from EBEWE PharmaGes. m. b. H (Utrecht, Austria); and Pr from Iran Hormone Company (Tehran, Iran).
Functional measurements
The levels of serum creatinine (Cr) and blood urea nitrogen (BUN) were measured using quantitative diagnostic kits (Pars Azmoon, Tehran, Iran). The serum and kidney levels of nitrite as a stable metabolite of nitric oxide (NO) were measured using a commercial kit (Promega Corporation, USA) that included the Griess reaction. The renal and serum levels of malondialdehyde (MDA) were measured using the manual method.[16]
Histology
The kidney specimens were fixed in neutral buffered formalin and processed for staining. Multiple sections were prepared from each animal for hematoxylin and eosin staining. The renal pathology index was evaluated by a pathologist who was blind to the study. According to severity of tubular lesions (hyaline cast, debris, vacuolization, flattening and degeneration of tubular cells, and dilatation of tubular lumen), kidney tissue damage score (KTDS) was graded from 0 to 4, 0 shows normal tubules without damage. The right kidney was homogenized for measurement of kidney nitrite and MDA levels.
Statistical analysis
Data were reported as mean ± standard error of mean comparison of the bodyweight, serum levels of BUN, Cr, MDA, and NO; and kidney weight (KW) were analyzed by one-way ANOVA followed by the least standard deviation test. Comparison between positive and negative control groups was performed by Student's t-test. Comparison of the groups with regard to the KTDS was carried out using Kruskal–Wallis and Mann–Whitney tests.
Results
The study was performed in two phases.
Phase 1
Effect of progesterone alone on serum levels of blood urea nitrogen and creatinine
Cisplatin-induced nephrotoxicity was evaluated by comparison of BUN and Cr levels between the positive and negative control groups. The levels of BUN (group 9: 156.54 ± 20.20; Group 10: 24.22 ± 3.37, P = 0.00) and Cr (Group 9: 2.86 ± 0.36; Group 10: 0.84 ± 0.05 P = 0.00) increased significantly in positive control (P < 0.05). The animals treated with Pr 2 and 5 mg/kg plus CP showed higher serum Cr level in comparison with the positive control group. Furthermore, the animals treated with Pr 5 mg/kg plus CP had higher serum Cr level when compared with Pr 2+CP, Pr 10+CP, and Pr 25+CP groups. The serum level of Cr was not significantly different between the animals treated with Pr 10 and 25 mg/kg plus CP when compared with the positive control group. Furthermore, we did not observe any significant difference in the serum level of BUN between Pr and CP-treated groups [Figure 1]. Also, no significant difference was seen in the serum levels of Cr and BUN between the group treated with Pr alone (Groups 1–4) and the negative control group [Figure 1].
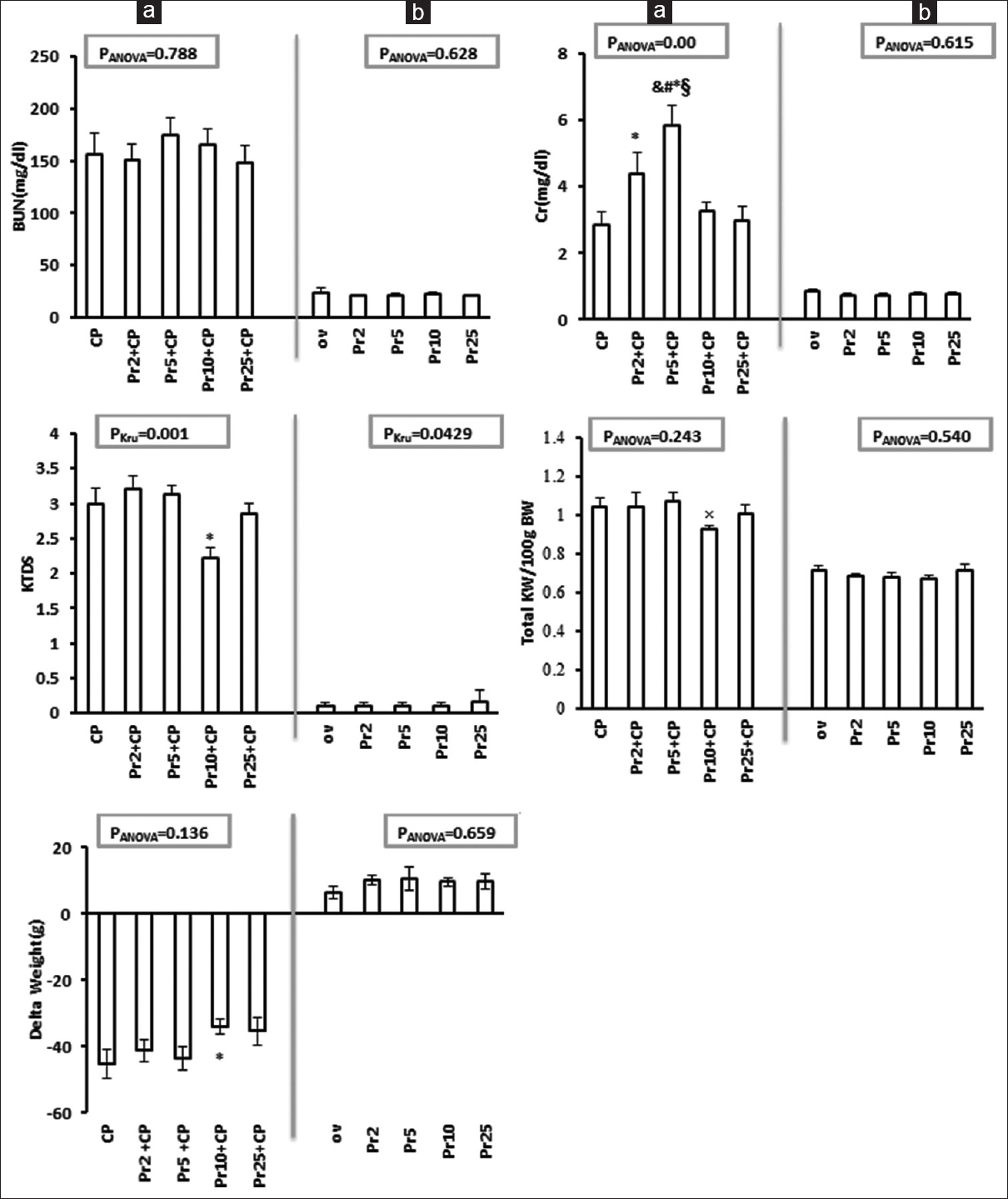
- Serum blood urea nitrogen (BUN) and creatinine, kidney tissue damage score, total kidney weight and weight change (delta weight) in all experimental groups (Phase 1). (a) The effect of progesterone on CP-induced nephrotoxicity. (b) The effect of progesterone (Pr) on the parameters measured. *, and, ×, §, and #indicate significant difference (P < 0.05) from the positive control (CP), Pr 2 + CP, Pr 5 + CP, Pr 10 + CP, and Pr 25 + CP, respectively
Effect of progesterone on kidney weight, kidney tissue damage score, bodyweight, and uterus weight changes
Both KW (Group 9: 1.03 ± 0.04; Group 10: 0.71 ± 0.02 P = 0.00) and KTDS (Group 9: 3 ± 0.21; Group 10: 0 ± 0 P = 0.001) increased and body weight (BW) (Group 9: −45.14 ± 4.46; Group 10: 6.5 ± 1.92 P = 0.00) decreased significantly in the positive control group compared with the negative control group (P < 0.05). KW (insignificantly) and KTDS (significantly, P < 0.05) declined in Pr 10+CP group when compared with the positive control group. BW loss in Pr 10+CP significantly decreased compared with the positive control group (P < 0.05) [Figure 1a]. Uterus weight (UW) (Group 9: 0.02 ± 0.00; Group 10: 0.01 ± 0.00 P = 0.02) increased significantly in the positive control group in comparison with the negative control group (P < 0.05), while the value decreased in Pr 10+CP group compared with the positive control, Pr 2+CP, and Pr 5+CP groups (P < 0.05) [Table 1a]. No difference was observed in BW, KTDS, KW, and UW between the group treated with Pr alone (Groups 1–4) and the negative control group [Figure 1b and Table 1b]. Accordingly, Pr at the dose of 10 mg/kg was considered as the most effective dose to reduced CP-induced nephrotoxicity.
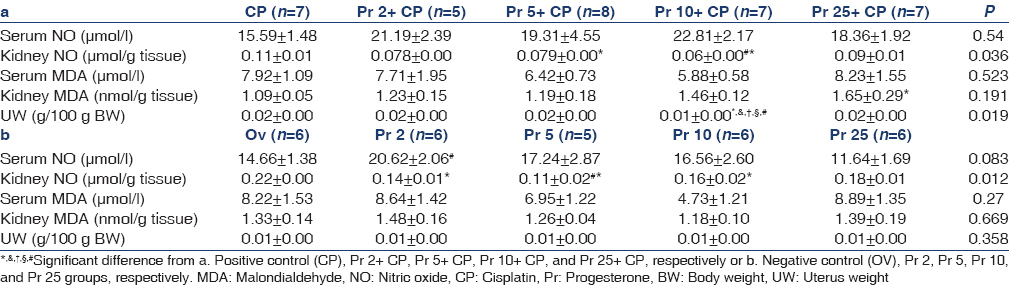
Effect of progesterone on kidney and serum levels of nitrite and malondialdehyde
No significant difference was observed between the positive and the negative control groups in the levels of serum nitrite (Group 9: 15.59 ± 1.48; Group 10: 14.66 ± 1.38 P = 0.664), serum MDA (Group 9: 7.92 ± 1.09; Group 10: 8.22 ± 1.53 P = 0.872) and kidney MDA (Group 9: 1.09 ± 0.05; Group 10: 1.33 ± 0.14 P = 0.117). In the positive control group the level of kidney nitrite (Group 9: 0.11 ± 0.01; Group 10: 0.22 ± 0.00 P = 0.00) decreased compared to the negative control group. No significant differences were observed in the serum levels of NO and MDA among the CP-treated groups. Pr at the doses of 5 and 10 mg/kg decreased kidney nitrite in comparison with the positive control group. Also, in the CP-treated groups, Pr 10 declined kidney nitrite in comparison with Pr 25. Kidney MDA increased significantly in Pr 25+CP group when compared with the positive control group [Table 1a]. Serum nitrite was significantly higher in Pr 2 compared to Pr 25 (P < 0/05). Kidney nitrite decreased at the doses of 2, 5, and 10 of Pr in comparison with the negative control group (P < 0/05). Serum MDA decreased in Pr 10 in comparison with the negative control group insignificantly [Table 1b].
Phase 2
Effect of estrogen and progesterone on serum levels of blood urea nitrogen and creatinine
The combination of Es/Pr reduced the levels of Cr and BUN in all the CP-treated groups compared with the positive control and Pr 10+CP groups [Figure 2].
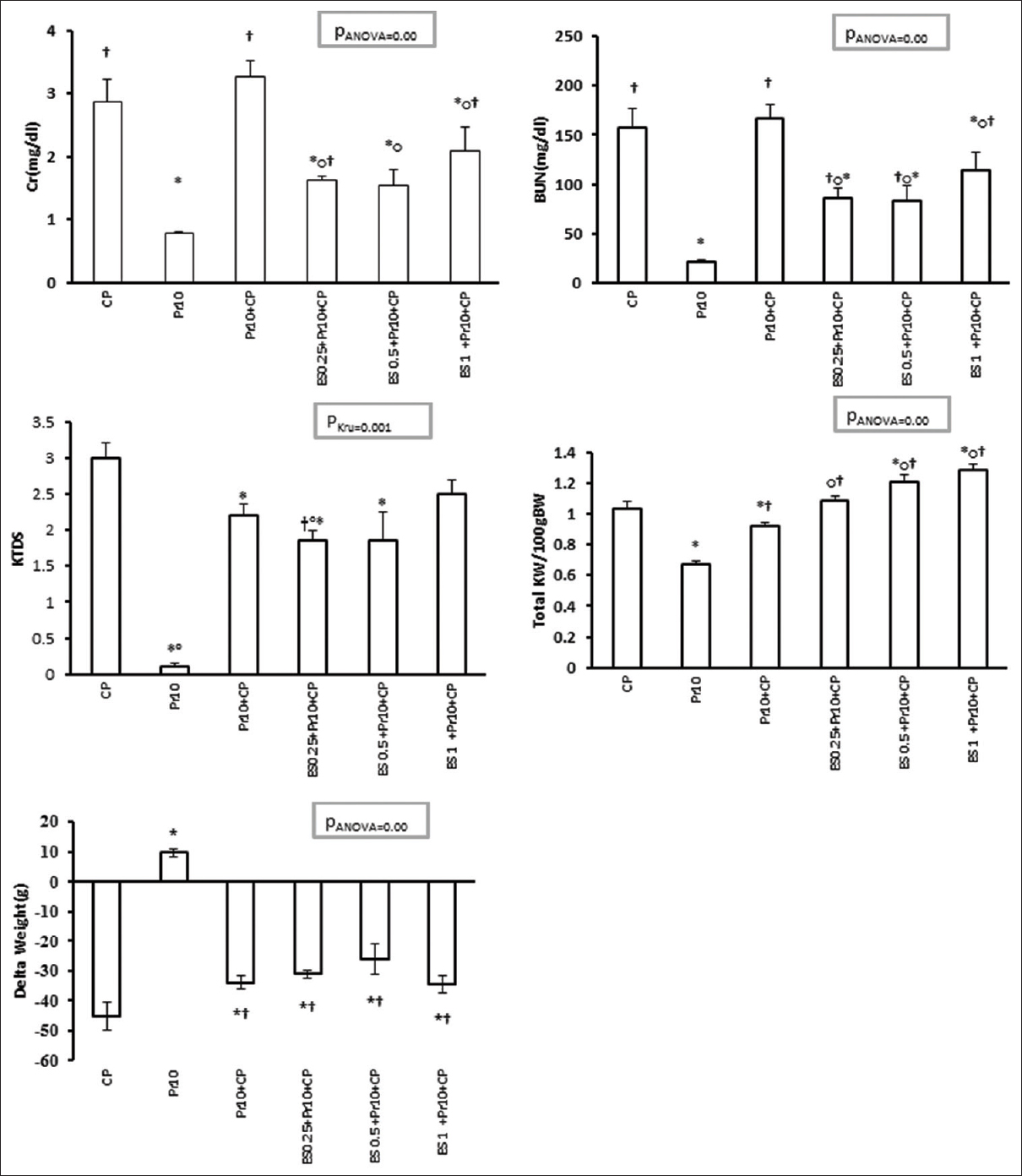
- Serum blood urea nitrogen and creatinine, kidney tissue damage score, total kidney weight, and delta weight in all experimental groups (Phase 2). The effect of co-administration Es/Pr on CP-induced nephrotoxicity. *, †, and o indicate significant difference from positive control (CP), Pr 10, and Pr 10+CP groups, respectively
Effect of progesterone on kidney weight, kidney tissue damage score, body weight, and uterus weight changes
KW increased in all Es/Pr-treated groups, and it was significant in Groups 12 and 13 compared with the positive control and Pr 10+CP groups (P < 0.05). KW decreased significantly in Pr 10+CP group in comparison with the positive control group and increased when compared with Pr 10 (P < 0.05) [Figure 2]. KTDS was significantly lower in Groups 11 and 12 in comparison with the positive control group (P < 0.05). Also, low dose of Es plus Pr declined KTDS significantly when compared to Pr 10+CP group (P < 0.05) [Figure 2]. BW loss was observed in all groups received CP in comparison with Pr 10 but Pr 10+CP and all Es/Pr-treated groups had a significantly smaller bodyweight loss when compared to the positive control group [Figure 2]. UW increased significantly in all the groups that received the combination of Es and Pr (P < 0.05) [Table 2].

Effect of progesterone on kidney and serum levels of nitrite and malondialdehyde
The level of serum nitrite increased in Group 12 (Pr 10+Es 0.5+CP) compared with the positive control group. Administration of CP increased serum MDA in the positive control group compared with Pr 10 group (P < 0.05). Serum MDA levels decreased significantly in all the groups that received Es plus Pr compared with the positive control group (P < 0.05). Administration of Es/Pr significantly increased the kidney level of MDA in Pr 10+Es 0.5 and Pr 10+Es 1 (P < 0/05). The kidney level of nitrite significantly reduced in all the CP-treated groups compared to Pr 10. Also, Pr 10+CP and Pr 10+Es 500+CP decreased kidney nitrite in comparison with the positive control group [Figure 3].
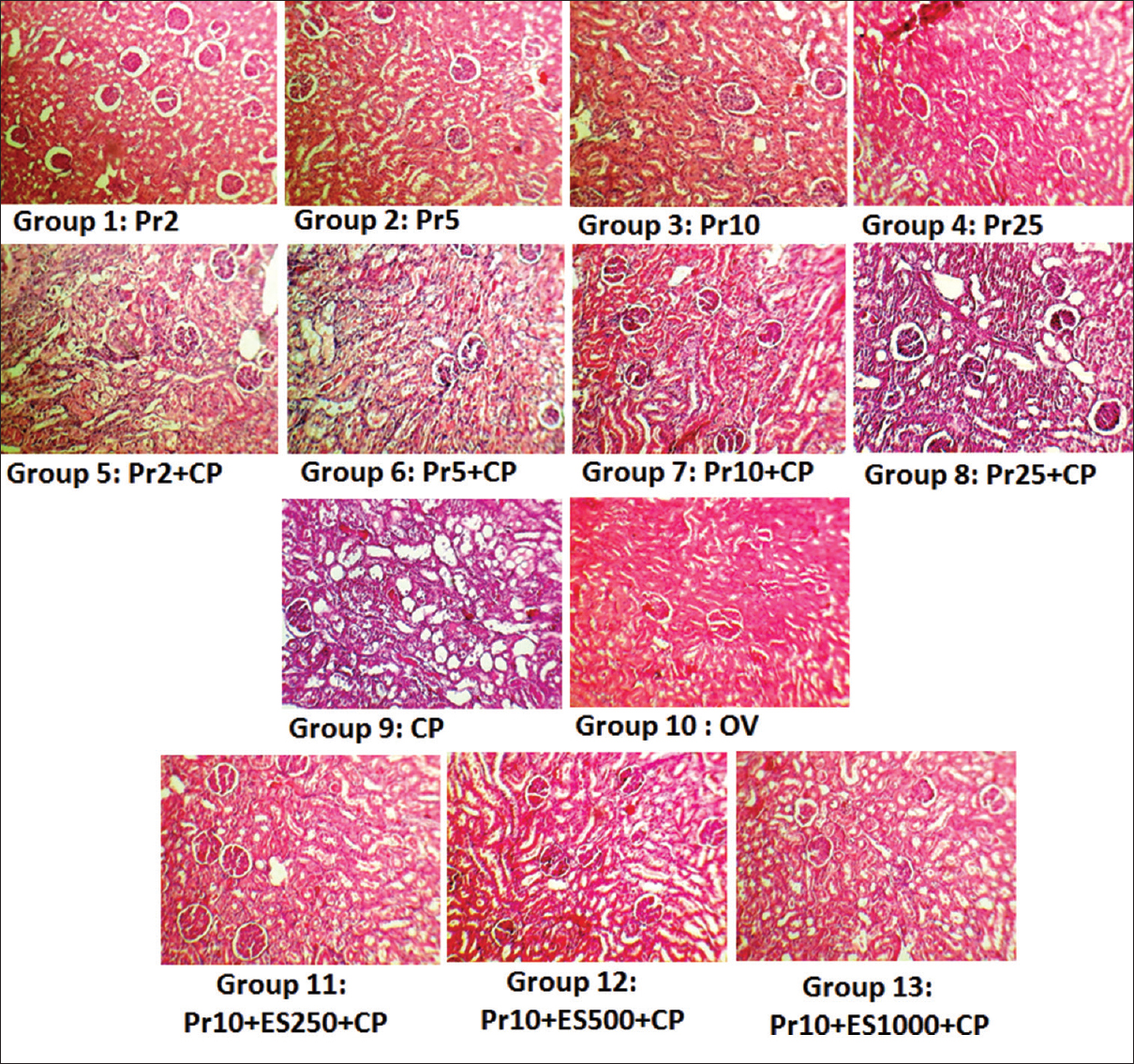
- Kidney tissue images (×100) in all the experimental groups. kidney tissue damage score was based on hyaline cast, debris, vacuolization, flattening and degeneration of tubular cells, and dilatation of tubular lumen and graded from 0 to 4. Groups 9 and 10 as positive and negative control groups showed degree of tubular damage 3 ± 0.22 and 0 ± 0 respectively. The arrows indicated tubular damage
Discussion
Our results demonstrated that Pr has a dose-dependent effect on reducing CP-induced nephrotoxicity. Also, combination of Es/Pr could decrease CP-induced nephrotoxicity. CP increased the serum levels of BUN and Cr, and decreased kidney nitrite. The KTDS and KW also increased, and weight loss was found in the positive control group. These findings were confirmed by previously published studies.[242021222324]
CP caused significant tubular damage[22] and oxidative stress involves in the kidney damage after CP treatment.[1] Kidney injury also happens due to increase in the level of caspases.[1] Moreover, CP reduced glomerular filtration rate (GFR), and therefore, the serum levels of BUN and Cr increased.[25] We observed that KW increased following administration of CP. This may be due to tubular damages and reduction in GFR, which increase accumulation water and salt in the kidney tissue.[2627] Weight loss in animals is caused by gastrointestinal complications due to CP injection.[4282930] Pr in high doses suppresses the production of pro-inflammatory agents such as IL-1 and TNF-alfa in monocyte,[17] and in low physiological concentrations increases IL-1 and superoxide production in rat and human macrophage.[31] Also, Pr could decrease inflammation by reduction of T cells and NK cells in macrophages.[3233] In addition, Pr can decrease ROS production that is a major cause of toxicity factor in CP-induced kidney injury.[34] Therefore, it seems improvement in some parameters such as KTDS, KW, BW, BUN and UW indicated a positive effect of Pr. In addition, Pr could decrease fas, fasl proteins expression and caspases 8, 3 and prevent apoptosis pathway,[3536] and possibly kidney damage is reduced due to a decrease in caspases level. In pregnancy, the ES/Pr increases GFR and renal blood flow and decrease BUN and Cr.[37] Grott et al. indicated that ES/Pr can prevent apoptosis through decrease caspases level and inflammation.[38] A surprising finding is that combination of Es/Pr increased KW that may due to increased cell proliferation by Es.[39] Also, Es/Pr combination increased UW probably because of growing and cell proliferation effect of female steroid hormones on the uterus.[40] Pr (10 mg/kg) or its combination with estradiol (0.05 mg/kg) increased the serum level of nitrite. In a previous study, it was demonstrated that Pr increases iNOS level and thus increases serum NO.[41] It was also shown that Es can increase eNOS level;[424344] therefore, Es/Pr combination could increase NO level. This finding was observed by the medium dose of Es as the effective dose but Es in high dose reduces iNOS expression level in rats.[45] In this study, reduction in kidney nitrite level in the presence of Pr was observed [Table 1]. CP also decreased kidney NO and combination of Pr and CP reduced kidney NO more than CP alone treated group and may be associated with effect of Pr on tissue NO production.[46] Incubation of endometrium with Pr decreased NO metabolite production in human endometrium.[47] Lipid peroxidation was monitored by examining the MDA level. MDA causes free radical damage to the cell membrane.[2223] Pr (25 mg/kg) increased serum and kidney MDA levels that is probably because high dose of Pr increased oxidative stress,[4849] and increased CP toxicity in ovarian cancer cells.[50] Es/Pr decreased serum MDA levels in Groups 11–13 because it was demonstrated that combination of Es/Pr decreased oxidation markers,[5152] but increased kidney MDA in Pr 10+Es 0.5 and Pr 10+Es 1 mg/kg groups, which is supported by other findings.[53]
Conclusion
Our results suggest that the beneficial effect of Pr on CP-induced nephrotoxicity is dose-dependent. Similarly, combination of Es/Pr strongly decreased CP-induced nephrotoxicity. In menopausal women who received hormone replacement therapy (HRT) and CP treatment simultaneously, maybe HRT help to reduce CP nephrotoxicity, and the reduction effect could relate to doses of Es and Pr.
Acknowledgment
This research was supported by Isfahan University of Medical Sciences.
Source of Support: This research was supported by Isfahan University of Medical Sciences
Conflict of Interest: The authors have no conflict of interests.
References
- Vitamin E, Vitamin C, or losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol. 2012;2012:284896.
- [Google Scholar]
- Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008;74:1538-47.
- [Google Scholar]
- The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389-96.
- [Google Scholar]
- Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy. 2009;55:399-406.
- [Google Scholar]
- Protective effects of Vitamin C on cisplatin-induced renal damage: A light and electron microscopic study. Ren Fail. 2008;30:1-8.
- [Google Scholar]
- Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004;19:348-55.
- [Google Scholar]
- N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren Fail. 2002;24:529-33.
- [Google Scholar]
- Sex-related difference in nitric oxide metabolites levels after nephroprotectant supplementation administration against cisplatin-induced nephrotoxicity in wistar rat model: The role of Vitamin E, Erythropoietin, or N-Acetylcysteine. ISRN Nephrol. 2013;2013:612675.
- [Google Scholar]
- Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2013;7:383-9.
- [Google Scholar]
- Gender difference in cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818-21.
- [Google Scholar]
- Idiopathic membranous nephropathy: The natural history of untreated patients. Kidney Int. 1988;33:708-15.
- [Google Scholar]
- Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol. 2000;11:319-29.
- [Google Scholar]
- Differential effects of 17beta-estradiol and of synthetic progestins on aldosterone-salt-induced kidney disease. Toxicol Pathol. 2009;37:969-82.
- [Google Scholar]
- Evidence against protective role of sex hormone estrogen in cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43-7.
- [Google Scholar]
- Effects of fennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol Int. 2013;20:138-45.
- [Google Scholar]
- Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098-104.
- [Google Scholar]
- Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724-33.
- [Google Scholar]
- Sex steroids and renal disease: Lessons from animal studies. Hypertension. 2008;51:976-81.
- [Google Scholar]
- Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47:1499-506.
- [Google Scholar]
- Selective reduction of cis-diamminedichloroplatinum (II) nephrotoxicity by ebselen. Cancer Res. 1990;50:7031-6.
- [Google Scholar]
- Effect of erythropoietin therapy on the progression of cisplatin induced renal injury in rats. Exp Toxicol Pathol. 2013;65:197-203.
- [Google Scholar]
- Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J Cancer Res Ther. 2008;4:111-5.
- [Google Scholar]
- Selective iNOS inhibition reduces renal damage induced by cisplatin. Toxicol Lett. 2008;176:48-57.
- [Google Scholar]
- Effect of cisplatin on renal function in rabbits: Mechanism of reduced glucose reabsorption. Toxicol Appl Pharmacol. 1995;130:19-26.
- [Google Scholar]
- A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J Res Med Sci. 2013;18:370-3.
- [Google Scholar]
- Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: Comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94-9.
- [Google Scholar]
- Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of cisplatin. Pharmacol Toxicol. 2003;92:300-3.
- [Google Scholar]
- The influence of relative body weight on toxicity of combination chemotherapy with cisplatin and etoposide. Cancer Chemother Pharmacol. 1998;42:386-90.
- [Google Scholar]
- Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435-59.
- [Google Scholar]
- Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442-50.
- [Google Scholar]
- Roles of reactive oxygen species in the regulation of luteal function. Rev Reprod. 1997;2:81-3.
- [Google Scholar]
- The effects of progesterone on apoptosis in the human trophoblast-derived HTR-8/SV neo cells. Mol Hum Reprod. 2007;13:869-74.
- [Google Scholar]
- Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum Reprod. 1996;11:980-5.
- [Google Scholar]
- Evaluation of changes in renal functions of pregnant women attending ante-natal clinic in Vom Plateau State, North-Central Nigeria. Arch Appl Sci Res. 2013;5:111-6.
- [Google Scholar]
- Progesterone and estrogen prevent cisplatin-induced apoptosis of lung cancer cells. Anticancer Res. 2013;33:791-800.
- [Google Scholar]
- Autocrine regulation of cell proliferation by estrogen receptor-alpha in estrogen receptor-alpha-positive breast cancer cell lines. BMC Cancer. 2009;9:31.
- [Google Scholar]
- Estrogen and progesterone receptors, cell proliferation, and c-fos expression in the ovine uterus during early pregnancy. Endocrinology. 1996;137:340-8.
- [Google Scholar]
- Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem. 2005;93:932-42.
- [Google Scholar]
- Effects of progesterone and estrogen on endothelial dysfunction in porcine coronary arteries. J Surg Res. 2005;124:104-11.
- [Google Scholar]
- Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001;280:H1699-705.
- [Google Scholar]
- Nitric oxide and hormones in breast cancer: Allies or enemies? Future Oncol. 2006;2(2):275-88.
- [Google Scholar]
- Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35:1309-16.
- [Google Scholar]
- Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin Pharmacol Toxicol. 2005;97:91-7.
- [Google Scholar]
- Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group bo×1 expression in human endometrium. Reprod Sci. 2008;15:559-66.
- [Google Scholar]
- Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782-9.
- [Google Scholar]
- Effect of estrogen, progesterone, LH, and FSH on oxidant and antioxidant parameters in rat uterine tissue. Int J Fertil Steril. 2009;3:119-28.
- [Google Scholar]
- Progesterone facilitates cisplatin toxicity in epithelial ovarian cancer cells and xenografts. Gynecol Oncol. 2008;110:251-5.
- [Google Scholar]
- Effects of estrogen, estrogen/progesteron combination and genistein treatments on oxidant/antioxidant status in the brain of ovariectomized rats. Eur Rev Med Pharmacol Sci. 2013;17:1869-73.
- [Google Scholar]
- Lipid peroxidation in ovariectomized and pinealectomized rats: The effects of estradiol and progesterone supplementation. Cell Biochem Funct. 2007;25:551-4.
- [Google Scholar]
- Effect of acute and chronic administration of progesterone, estrogen, FSH and LH on oxidant and antioxidant parameters in rat gastric tissue. Chem Biol Interact. 2009;182:1-6.
- [Google Scholar]







