Translate this page into:
Association of chemokine receptor CX3CR1 V249I and T280M polymorphisms with chronic kidney disease
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The chemokine fractalkine (CX3CL1) and its receptor CX3CR1 are involved in the activation of leukocytes. Two common single-nucleotide polymorphisms of the CX3CR1 gene, V249I and T280M, have been associated with reduced fractalkine signaling, leading to decreased adhesive function and leukocyte chemotaxis. We hypothesized that variation in the CX3CR1 gene could be associated with chronic kidney disease (CKD), a disease of inflammatory activation. We studied the association between CX3CR1 V249I and T280M polymorphisms, and fractalkine and highly sensitive C-reactive protein (hs-CRP) levels in 123 patients with CKD and 100 healthy controls (HCs). Genotype analysis was done by polymerase chain reaction-restriction fragment length polymorphism, and fractalkine and hs-CRP levels were analyzed by enzyme-linked immunosorbent assay. MM genotype of T280M was absent in CKD patients, while in controls it was seen in 1% of the individuals. The allele frequencies in both the groups were similar (P = 0.059). Compared to HC, M280M + T280M genotype was more frequent in CKD (P = 0.041). The frequency of II genotype of V249I was 0.8% in CKD, whereas in HC, it was 2%. I249I + V249I genotype was more frequent in CKD as compared to HC (P = 0.034). No difference in allelic frequency of V249I was noted between the two groups (P = 0.061, odds ratios = 1.74, 95% confidence intervals = 0.96–3.12). Plasma fractalkine and serum hs-CRP levels were higher in CKD subjects (P = 0.004 and P < 0.0001). No association of either genotype was found with fractalkine and hs-CRP levels. Polymorphisms at I249 and M280 genotype in CX3CR1 gene are associated with CKD; however, there was no association of fractalkine or inflammatory marker with these genotypes.
Keywords
Chemokine receptor 1
chronic kidney disease
fractalkine
inflammation
single-nucleotide polymorphism
Introduction
Chronic kidney disease (CKD) is associated with persistent inflammatory activation. The adverse outcomes of CKD include progression to end-stage renal disease (ESRD) and increased risk for cardiovascular disease (CVD).[1] Increased circulating inflammatory proteins are powerful predictors of all-cause mortality and cardiovascular death in ESRD patients.[12] Inflammation involves complex interactions among immune cells and soluble proteins such as cytokines, chemokines, and co-stimulatory molecules in response to several stimuli.[3]
Chemokine fractalkine (CX3CL1), a member of the CX3C chemokine subfamily, is a transmembrane molecule expressed on endothelial cells. It is activated by pro-inflammatory cytokines[4] and serves as an adhesion protein promoting the retention of monocytes and T-cells. It also exists in a soluble form, which resembles a conventional chemokine and induces chemotaxis. CX3C chemokine receptor 1 (CX3CR1), a 7-transmembrane domain G-protein-coupled receptor, is expressed by natural killer cells, monocytes, and CD8+ T-cells and was recently shown on CD4+ T-cells.[56] Both chemotaxis and adhesion are mediated by CX3CR1-fractalkine signaling. Accumulating evidence indicates that CX3CL1-CX3CR1 pathway is involved in the pathogenesis of inflammatory diseases,[7] including atherosclerotic CVD in CKD.[689]
Fractalkine and its receptor CX3CR1 have been suggested to play a key role in human and animal model of renal disease. Fractalkine expression and cells expressing CX3CR1 have been shown in renal abnormalities.[1011121314] A report by Feng et al.[15] shows that anti-CX3CR1 antibody treatment inhibits leukocyte infiltration into the glomeruli, improving renal function. Another study[16] showed that CX3CR1 inhibition is protective against ischemic acute renal failure in mice. These studies suggest a role for fractalkine/CX3CR1 axis in pathogenesis of human renal disease.
The two most common single-nucleotide polymorphisms (SNPs) in the CX3CR1 gene (V249I and T280M) lead to changes in conserved amino acids in the receptor protein. Both polymorphisms are in strong linkage disequilibrium, forming a common I249M280 haplotype.[17] An association of several inflammatory diseases including arteriosclerosis and coronary artery disease and ESRD with these SNPs has been demonstrated.[18192021] The early genotyping of CX3CR1 polymorphism may play a role in identifying advanced ESRD progression and potential use of anti-CX3CR1 therapy.[18]
In view of the existing studies suggesting a role of this pathway in complications in CKD, we studied the frequency of V249I and T280M polymorphisms in the CX3CR1 gene and evaluated its association with circulating fractalkine level and highly sensitive C-reactive protein (hs-CRP).
Materials and Methods
Patients
A total of 123 CKD patients with estimated glomerular filtration rate (eGFR) <30 ml/min were recruited for the study from Nephrology Department of Postgraduate Institute of Medical Education and Research, Chandigarh. Patients below 18 and above 70 years of age, those with malignancy, or infection in the last 3 months were excluded. The Institute Ethics Committee approved the study protocol and all subjects provided a written consent. One hundred healthy age-, sex-, and ethnicity-matched North Indians were selected as controls. All study subjects underwent a thorough clinical examination. Hypertension and diabetes were diagnosed according to the standard criteria. Details of clinical features and laboratory investigations were recorded.
Genetic analysis
Genomic DNA was extracted from cells in the buffy coat isolated from samples collected after overnight fasting using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. V249I and T280M polymorphisms were detected using polymerase chain reaction (PCR) followed by restriction fragment length polymorphism. Details of the location of the SNPs, primer sequences, PCR conditions, and restriction enzymes (New England BioLabs Inc., USA) with product sizes are given in Table 1. The digested PCR products were resolved on 2–3% agarose gels stained with ethidium bromide [Figure 1].

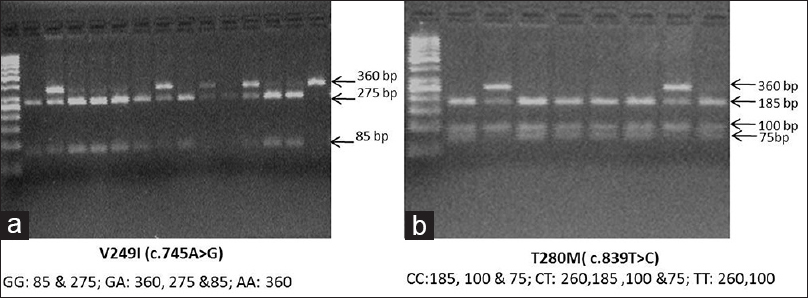
- Representative image of agarose gel electrophoresis of (a) CX3CR1 V249I and (b) CX3CR1 T280M polymorphism
Highly sensitive C-reactive protein and fractalkine level
Sandwich enzyme-linked immunosorbent assay was used to determine hs-CRP (Diagnostics Biochem Canada Inc., London, Ontario, Canada) and circulating fractalkine (Ray Biotech Inc., Norcross, GA, USA) levels in plasma according to the manufacturer's instructions.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed using SPSS 16.0 (SPSS Chicago, IL, USA). Differences between groups were analyzed with the Student's t-test when the variable was normally distributed. Mann–Whitney U-test was used for data with skewed distribution. Hardy–Weinberg equilibrium (HWE) was tested for each SNP for cases and controls. Allelic and genotypic associations of SNPs were evaluated by Pearson's χ2-test; odds ratios (OR) and 95% confidence intervals (CI) were determined. Haplotype analysis was done using online software SHEsis.[22] All P values were calculated two-sided, and a value of <0.05 was considered significant.
Results
General demographic and clinical characteristics of the study population are shown in Table 2. A majority of the patients were males. As expected, the blood pressure was higher among the patients. About 33% of the CKD patients were diabetic and 85% had hypertension.

Plasma fractalkine and serum hs-CRP levels were increased in subjects with CKD as compared to healthy controls (438.6 ± 490.6 vs. 239.7 ± 73.1 pg/ml, P = 0.004 and 94.4 ± 58.9 vs. 48.4 ± 52.9 µg/ml, P < 0.0001 respectively).
Genotype frequencies in cases and controls were in HWE (for T280M; P = 0.097, χ2 = 2.750 in CKD and P = 0.528, χ2 = 0.397 in HC), for V249I; P = 0.181, χ2 = 1.784 in CKD and P = 0.201, χ2 = 1.630 in HC).
The distribution of genotype and allele frequencies for CX3CR1 T280M and V249I polymorphisms are shown in Table 3. For the 280 locus, the allele frequencies in both groups were similar (P = 0.059, OR = 1.84, 95% CI = 0.96–3.51). Compared to HC, the MM and TM variants were more frequent in CKD (P = 0.041, OR = 2.16, 95% CI = 1.07–4.32). At the 249 position, II and VI variants were more frequent in CKD as compared to HC (P = 0.034; OR = 2.1, 95% CI = 1.09–4.01). No difference in allelic frequency, however, was noted between the two groups (P = 0.061, OR = 1.74, 95% CI = 0.96–3.12).

The haplotype distribution is shown in Table 4. The V249T80 haplotype was more frequent in healthy subjects. CKD subject shows increased IT and IM haplotypes but the difference was not significant (P = 0.08, OR = 2.7 and P = 0.128, OR = 1.7, respectively).

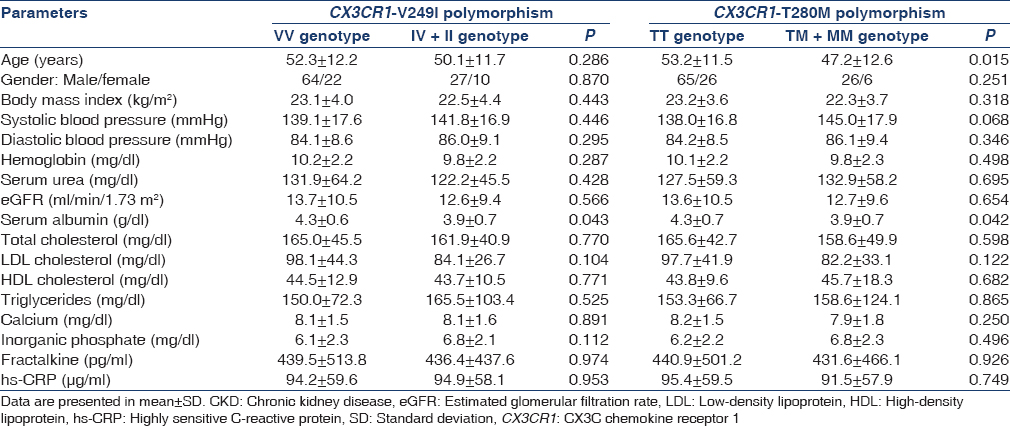
We also compared the clinical characteristics of CKD subjects according to their genotypes, but found no significant association with any parameter except serum albumin [Table 5]. IV + II and TM + MM had lower serum albumin levels as compared to VV and TT, respectively (P = 0.043 and P = 0.042).
Discussion
Persistent inflammatory activation has been identified as the key factor behind development and progression of CVD in patients with CKD. Why some patients show more intense inflammatory activation and others do not is not well understood. One possibility is differential activation secondary to genetic variations in pro-inflammatory cytokine/chemokine and their receptors. We have shown an increase in CD4+ CX3CR1+ T-cell population and plasma fractalkine level in advanced renal failure patients'. In another study, the CX3CR1 V249I SNP was shown to be associated with advanced CKD.[18]
Epidemiological and clinical study reports have investigated the association between polymorphisms in the CX3CR1 and coronary artery disease,[2123] risk of acute rejection in renal transplant recipients,[24] cancer rates in renal transplant recipient,[25] age-related macular degeneration,[26] and HIV.[17] The presence of V249 and T280 SNPs in CX3CR1 gene leads to its overexpression by activated inflammatory cells and with higher binding affinity for fractalkine.[1727] Although the exact mechanism is not clear, it is important that CX3CR1 has been reported to function in vitro in CD4 as an HIV co-receptor, and the M280 allele is associated with reduced receptor expression on PBMCs from HIV-positive subjects.[17] In contrast to HIV, in which disease progression was faster in M280 homozygotes, the association of CX3CR1 with CKD was noted for I249 and M280 heterozygotes, whereas only I249 was associated with ESRD in previous report.[18] These report suggest a differential effect of the two polymorphisms on CX3CR1 function.
The association between fractalkine level and genetic variants of its receptor CX3CR1 has not been investigated so far. Although patients with CKD exhibit increased levels of plasma fractalkine, no association with CX3CR1 V249I and T280M was noted. Further, there was no association of these polymorphisms with other parameters including hs-CRP. However, decrease in serum albumin was noted in genotypes with I249 and M280. However, in relation to coronary artery disease, M280 and I249 alleles seem to be protective, perhaps due to a reduced number of receptor binding sites and a decreased fractalkine binding affinity.[192021]
There are some limitations to this study. The number of cases is relatively small. Also, it is likely that other genetic factors might play a role in inflammatory activation. Ideally, therefore, interaction between the various factors needs to be evaluated. On the plus side, it is well-selected homogenous population with well-characterized subjects, reducing phenotyping errors and bias. The study also includes well-matched controls of the same ethnic background, which reduced the risk of population stratification that is one of the major causes of false results in case–control studies. To conclude, our results suggest that CX3CR1 V249M and T280M polymorphisms may influence the risk of CKD development. These polymorphisms could be added to the list of potential markers to enable physicians in determining the CKD risk profile. The study, however, needs to be replicated in a larger population with longitudinal follow-up study. The ongoing Indian chronic kidney disease study will provide such an opportunity.
Financial support and sponsorship
This study was funded by a grant from the Indian Council of Medical Research, Government of India, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
References
- Increased circulating inflammatory proteins predict a worse prognosis with valvular calcification in end-stage renal disease: A prospective cohort study. Am J Nephrol. 2008;28:647-53.
- [Google Scholar]
- Troponin T, left ventricular mass, and function are excellent predictors of cardiovascular congestion in peritoneal dialysis. Kidney Int. 2006;70:444-52.
- [Google Scholar]
- Fractalkine in vascular biology: From basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24:34-40.
- [Google Scholar]
- Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521-30.
- [Google Scholar]
- Association of circulating fractalkine (CX3CL1) and CX3CR1(+) CD4(+) T cells with common carotid artery intima-media thickness in patients with chronic kidney disease. J Atheroscler Thromb. 2011;18:958-65.
- [Google Scholar]
- Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568-81.
- [Google Scholar]
- Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus-and activation-regulated chemokine, are expressed in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:923-9.
- [Google Scholar]
- Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1) Circulation. 2003;108:2498-504.
- [Google Scholar]
- Fractalkine expression on human renal tubular epithelial cells: Potential role in mononuclear cell adhesion. Clin Exp Immunol. 2002;129:150-9.
- [Google Scholar]
- Upregulation of fractalkine in human crescentic glomerulonephritis. Nephron. 2001;87:314-20.
- [Google Scholar]
- Fractalkine expression and the recruitment of CX3CR1 cells in the prolonged mesangial proliferative glomerulonephritis. Kidney Int. 2002;61:2044-57.
- [Google Scholar]
- Expression of the fractalkine receptor (CX3CR1) in human kidney diseases. Kidney Int. 2002;62:488-95.
- [Google Scholar]
- Prevention of crescentic glomerulonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int. 1999;56:612-20.
- [Google Scholar]
- Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008;294:F264-71.
- [Google Scholar]
- Rapid progression to AIDS in HIV individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274-7.
- [Google Scholar]
- Chemokine (CCR) and fractalkine (CX3CR) receptors and end stage renal disease. Inflamm Res. 2011;60:399-407.
- [Google Scholar]
- Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241-50.
- [Google Scholar]
- Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401-7.
- [Google Scholar]
- Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925-8.
- [Google Scholar]
- A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio-x.cn) Cell Res. 2009;19:519-23.
- [Google Scholar]
- Association of V249I and T280M polymorphisms in the chemokine receptor CX3CR1 gene with early onset of coronary artery disease among North Indians. Genet Test Mol Biomarkers. 2012;16:756-60.
- [Google Scholar]
- Chemokine receptor polymorphism and risk of acute rejection in human renal transplantation. J Am Soc Nephrol. 2002;13:754-8.
- [Google Scholar]
- Influence of fractalkine receptor gene polymorphisms V249I-T280M on cancer occurrence after renal transplantation. Transplantation. 2013;95:728-32.
- [Google Scholar]
- Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1-18.
- [Google Scholar]







