Translate this page into:
Anti-glomerular basement membrane crescentic glomerulonephritis: A report from India and review of literature
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anti-glomerular basement membrane (anti-GBM) disease is an autoimmune disease that most commonly presents as rapidly progressive glomerulonephritis with or without pulmonary involvement. It is characterized by the presence of antibodies directed to antigenic targets within glomerular and alveolar basement membranes. This study was performed to evaluate the clinicopathological features and outcome in anti-GBM crescentic glomerulonephritis (CrGN) at a tertiary care center in North India over a period of 9 years (January 2004 to December 2012). A diagnosis of anti-GBM CrGN was made in the presence of >50% crescents, linear deposits of IgG along GBM, and raised serum anti-GBM antibody titer. Of 215 cases of CrGN diagnosed during this period, 11 had anti-GBM CrGN. Anti-GBM CrGN was found at all ages but was most common in the third to fifth decade with no gender predilection (mean age 48 +/- 15 years, 13–67 years). Patients presented with a mean serum creatinine of 10.2 +/- 5.3 mg/dl and sub-nephrotic proteinuria. Pulmonary involvement was present in two patients. Myeloperoxidase-antineutrophil cytoplasmic antibody was positive in two (2/11) elderly patients. Follow-up was available in four patients for a range of 30-270 (mean 99.5 ± 114.5) days, two remained dialysis dependent while two died due to uremia and sepsis. Our findings show that anti-GBM disease is a rare cause of CrGN in India, accounting for only 5% of patients. It usually presents as a renal-limited disease and is associated with a poor renal outcome.
Keywords
Anti-glomerular basement membrane antibody
anti-glomerular basement membrane disease
crescentic glomerulonephritis
rapidly progressive glomerulonephritis
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is a rare autoimmune disorder characterized by circulating auto-antibodies against epitopes in the α3-chain of type IV collagen present in the glomerular and/or pulmonary basement membranes.[1] It is responsible for 10-20% of rapidly progressive renal failure.[2] The renal biopsy shows crescentic glomerulonephritis (CrGN) >50% glomeruli with crescents) in more than 80% of patients.[3456] However, CrGN due to anti-GBM disease accountis for only 10-20% of patients, as compared to the other causes namely immune-complex-mediated and pauci-immune CrGN.[3] Pulmonary renal syndrome (Goodpasture syndrome) is more common in young patients with anti-GBM disease.[78]
Despite the availability of potent immunosuppressive agents, less than one-third patients of anti-GBM disease have a preserved kidney function after 6 months.[9] The survival is even more dismal in patients presenting with rapidly progressive renal failure.
We report our experience of anti-GBM CrGN over 9 years from a tertiary care center in North India.
Materials and Methods
Renal biopsies diagnosed as anti-GBM CrGN at the Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow over a period of 9 years (January 2004 to December 2012) were included in this study. Only patients with renal biopsies for both light microscopy and for immunofluorescence were included. Diagnosis of anti-GBM CrGN required >50% crescents, linear IgG deposition along GBM on immunofluorescence and elevated serum anti-GBM antibody titers. The study was approved by the Institutional Ethics Committee.
Clinical presentation and investigations at the time of biopsy including hematology, biochemistry, urine examination, serum complement levels, and serology for autoantibodies were noted. Clinical records were reviewed to determine the serum creatinine and proteinuria at follow-up.
Biopsies were performed under ultrasound guidance by a spring loaded biopsy gun using 18G needle under local anesthesia. Separate cores were received for light microscopy and immunofluorescence. For light microscopy, serial sections of 3 μm thickness were obtained from renal biopsy received in 10% buffered formalin. All biopsies were stained with Hematoxylin and Eosin, Periodic Acid Schiff, Periodic Silver Methenamine, and Masson's trichrome. Biopsies were evaluated for the percentage of glomeruli showing glomerular sclerosis, increased mesangial cellularity, endocapillary proliferation, neutrophilic exudation, fibrinoid necrosis, and crescent formation. Tubular atrophy, interstitial inflammation, and interstitial fibrosis were graded semi-quantitatively as 0-3 + based on the percentage of cortical involvement (0 – none; 1+ – <25%; 2+ – 25-50%; 3+ – >50%). Blood vessel changes were recorded.
Biopsy was transported in normal saline for immunofluorescence, and immediately processed by immersing in isopentane and snap freezing in liquid nitrogen. The biopsies were sectioned at 5 μm thickness in a cryostat at −20°C. All biopsies were stained with fluorescein isothiocyanate conjugated antibodies for IgG, IgM, IgA, C3, and C1q (Dako, Denmark) at 1:60 dilution, mounted in buffered glycerol and viewed under Nikon Eclipse 80i immunofluorescence microscope. The location, pattern, and intensity (on a scale of 0–4+) of positive immunostaining was recorded
Statistical analysis
Survival analysis was performed using Kaplan-Meier curves and differences in renal survival were tested using the log-rank test. SPSS version 15.0 (IBM, Chicago, SPSS Inc.) was used for statistical analysis and P < 0.05 was considered as significant.
Results
Over a period of 9 years (January 2004 to December 2012), 215 biopsies were diagnosed as CrGN, of which 11 were diagnosed as anti-GBM nephritis (5.1%). Table 1 summarizes the clinical and laboratory data.
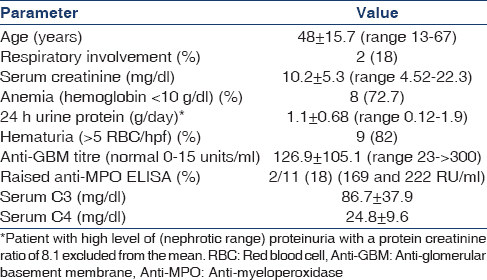
Most patients were in the third to fifth decade with no sex predilection (M:F 5:6). The most common renal presenting symptom was oliguria (n = 10; 83.3%) and extrarenal symptom was cough (n = 2; 16.6%). Eight patients had hypertension and two presented with cough and hemoptysis. X-ray revealed pulmonary hemorrhage in both these patients. All patients presented as a rapidly progressive renal failure with a high serum creatinine and were dialysis-dependent at the time of presentation.
Only one patient had nephrotic proteinuria. In rest, the proteinuria was subnephrotic with 24 hr urinary protein ranging from 0.12 to 1.9 gmg/day. The serum C3 and serum C4 levels were within normal range in all patients. Serum anti-GBM titers was high in all patients. Antineutrophil cytoplasmic antibody (ANCA) screening done in all patients was positive in two patients (18%) with raised anti-myeloperoxidase (MPO) titers.
Histological features
The renal biopsies in anti-GBM nephritis had an average of 13 glomeruli (range 6–19). Ten or more glomeruli were available in 66.7% of the biopsies.
Global glomerulosclerosis involved a mean of 19% glomeruli (range 14–66%). Crescents were found in 53-100% of unsclerosed glomeruli (76% ± 19%). The crescents were predominantly fibrocellular (65% ± 32%) followed by cellular (30%) and fibrous (5%) crescents [Figure 1].
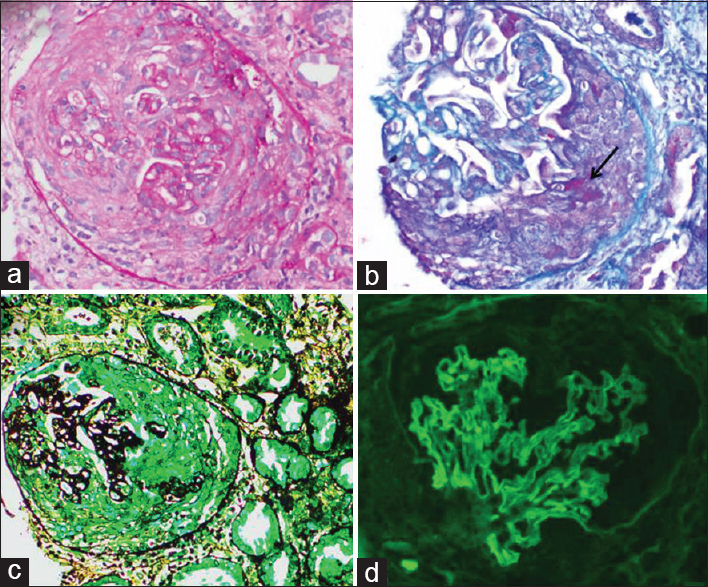
- Renal biopsy in anti – glomerular basement membrane crescentic glomerulonephritis showing a cellular crescent obliterating the Bowman space with compression of the glomerular tuft and presence of fibrinoid material in the crescent (B – arrow); immunofluorescence for IgG shows linear staining along glomerular capillary wall (d). (a) H and E; (b) mason trichrome; (c) periodic acid silver methenamine; (d) fluorescein isothiocyanate conjugated anti – immunoglobulin G, ×200
Mesangial and endocapillary proliferation was seen in an average of 25% (range 23–35%) and 41.6% (range 35–49%) glomeruli, respectively. Fibrinoid necrosis involving the glomerular capillary tuft was noted in 6 (54.5%) renal biopsies. Rupture of Bowman's capsule was seen in 7 (63.6%) renal biopsies, however, periglomerular granulomatous inflammation was uncommon, found in only one renal biopsy. Tubular atrophy was seen in all biopsies, being mild in nine biopsies (82%). Mild interstitial fibrosis was seen in six biopsies (64.5%).
On immunofluorescence, all renal biopsies showed linear strong (3–4+) IgG deposition along the GBM [Figure 1d]. Two renal biopsies showed IgA (1–2+) along with IgG deposition. Associated C3 complement deposits were seen in seven renal biopsies.
The treatment protocol followed for patients of CrGN in all patients was intravenous methylprednisolone 750 mg followed by oral prednisolone 0.5 mg/kg for 4 weeks and oral cyclophosphamide (1.5 mg/kg). Oral prednisolone was tapered by 2.5 mg/day every 2 weeks.
All cases were dialysis-dependent at the time of presentation. Follow-up was available in four patients for 30–270 days (mean ± SD 99.5 ± 114.5 days; median 49 days). Two patients (82%) remained dialysis dependent on follow-up while two died due to septicemia and uremia after a period of 9 months and 10.2 months, respectively.
Discussion
Anti-GBM nephritis is an important pathologic correlate of rapidly progressive renal failure. This study included 11 patients of anti-GBM nephritis evaluated at a single center over a period of 9 years.
Studies from the North America and Western Europe have shown that anti-GBM disease accounts for 10–15% of CrGN.[31011] An incidence of 6–16% has been reported from China and Japan.[12131415] Reports from India are variable. A study from North-East India including 34 patients of CrGN over a period of 3.5 years reported an incidence of 14.7%.[14] However, another study from North India found no case of anti-GBM nephritis over a 2 years study of 46 patients of CrGN.[16] We found a prevalence of 5% for anti-GBM nephritis among 215 patients diagnosed as CrGN on renal biopsy.
Reports from China have reported bimodal peaks of onset for anti-GBM nephritis, one in the second to third and other in the sixth to seventh decade.[8] An earlier study from China of 172 patients of CrGN over a 16 years period reported an earlier age of onset (mean ± SD 27.8 ± 7.6 years) for anti-GBM nephritis (n = 15).[12] In one of the largest series, Jennette et al. reported the mean age of onset for anti-GBM nephritis in the third to sixth decade (mean ± SD 52 ± 21 years).[317] We also found the similar age of onset with most patients (90%) presenting in the fourth decade (mean ± SD 48 ± 15.7 years). Anti-GBM glomerulonephritis is rare in children.[18] Our study included only one patient of pediatric anti-GBM nephritis. Cui et al. found 22.6% elderly patients (65 years or older) in 221 consecutive Chinese patients with anti-GBM disease diagnosed in a 10 years period.[7] Our study included two patients aged >65 years (2/11; 18%).
The pulmonary renal syndrome is reported in 40–60% patients with anti-GBM disease.[319] We observed pulmonary renal syndrome in only two patients (16.6%). Less pulmonary involvement has been reported in elderly patients with anti-GBM disease who have a milder disease as compared to young patients.[7]
Anti-GBM nephritis usually presents as a rapidly progressive renal failure. Studies have reported 40-80% incidence of renal insufficiency in anti-GBM disease.[192021] In patients with rapidly progressive glomerulonephritis, Jennette et al. found raised serum creatinine at a presentation in most patients of anti-GBM nephritis (mean 9.7 ± 7.2 mg/dl).[3] All our patients had renal insufficiency as the study included patients of anti-GBM disease with CrGN. Proteinuria is usually in the subnephrotic range in anti-GBM nephritis.[3] We also found similar results.
Co-existence of anti-GBM and ANCA has been reported in one-third to one-fourth patients of anti-GBM disease.[3819] We found MPO-ANCA in two of our patients (16.6%). Alchi et al., found that dual-positive patients of anti-GBM nephritis were almost 20 years older than those with anti-GBM positivity alone.[21] Both of our patients were also elderly, both being in the in the geriatric age group (>60 years). A similar incidence was reported in an earlier study from China.[19] Although, presence of “double positivity” for ANCA and anti-GBM antibodies in anti-GBM disease is not associated with renal prognosis, it has been reported to be associated with increased incidence of extrarenal symptoms in CrGN.[822] However, none of our patients with double positivity had extrarenal involvement.
On immunofluorescence, IgG is the most common immunoglobulin and C3, the most common complement with linear deposits along GBM.[312] Tang et al. had reported IgG in 77.5% of patients and C3 in (21.1%) cases of anti-GBM nephritis.[12] We found linear IgG in all and C3 deposition in 58.3% cases.
The renal prognosis in anti-GBM disease is usually poor with only less than one-third patients having maintained kidney function after 6 months of follow-up.[9] Our study and earlier reports from India suggest that these patients are diagnosed late resulting in a poor renal outcome.[14] The poor response to therapy may be attributed to the presence of rapidly progressive glomerulonephritis in all our patients who were dialysis-dependent at the time of presentation. Alchi et al. reported oligo-anuria and percentage of glomerular crescents as the strongest predictor associated with poor renal outcome in anti-GBM disease.[21] Other studies have shown that patients of anti-GBM disease presenting with rapidly progressive glomerulonephritis and high serum creatinine at presentation have a poor renal outcome.[15232425] Similar results have been reported in a large study from Australia and New Zealand (ANZDATA Registry).[26] In this study, between 1963 and 2010, 449 patients (0.8%) with anti-GBM disease on renal replacement therapy for end-stage renal disease were identified. The median survival on dialysis was 5.9 years. Only 13 patients recovered renal function; however, ten subsequently experienced renal death after a median period of 1.05 years.[26] Despite aggressive immunosuppression, the response in anti-GBM disease is seen only when the disease is detected at an early stage, making a high degree of awareness necessary to find these rare cases.
Our findings show that anti-GBM nephritis usually presents as a renal-limited disease. It is associated with a poor renal outcome as most patients present with advanced renal failure. Early diagnosis before the development of advanced disease may help in improving the outcome of these patients. Newer modalities of treatment need to be developed for this aggressive disease with a poor renal survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Advances in the genetics of anti-glomerular basement membrane disease. Am J Nephrol. 2010;32:482-90.
- [Google Scholar]
- Antiglomerular basement membrane antibody-mediated glomerulonephritis and Goodpasture's syndrome. Medicine (Baltimore). 1979;58:348-61.
- [Google Scholar]
- Management of idiopathic crescentic and diffuse proliferative glomerulonephritis: Evidence-based recommendations. Kidney Int Suppl. 1999;70:S33-40.
- [Google Scholar]
- Clinical features and outcomes of anti-glomerular basement membrane disease in older patients. Am J Kidney Dis. 2011;57:575-82.
- [Google Scholar]
- ANCA-associated vasculitis and anti-GBM disease: The experience in China. Nephrol Dial Transplant. 2010;25:2062-5.
- [Google Scholar]
- Diagnosis and classification of Goodpasture's disease (anti-GBM) J Autoimmun. 2014;48-49:108-12.
- [Google Scholar]
- Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033-42.
- [Google Scholar]
- The prognostic significance in Goodpasture's disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract. 2003;94:c59-68.
- [Google Scholar]
- Clinical spectrum of diffuse crescentic glomerulonephritis in Chinese patients. Chin Med J (Engl). 2003;116:1737-40.
- [Google Scholar]
- A nationwide survey of rapidly progressive glomerulonephritis in Japan: Etiology, prognosis and treatment diversity. Clin Exp Nephrol. 2009;13:633-50.
- [Google Scholar]
- Clinicopathologic spectrum of crescentic glomerulonephritis: A hospital-based study. Saudi J Kidney Dis Transpl. 2014;25:689-96.
- [Google Scholar]
- Anti-glomerular basement membrane antibody disease in Japan: Part of the nationwide rapidly progressive glomerulonephritis survey in Japan. Clin Exp Nephrol. 2008;12:339-47.
- [Google Scholar]
- Crescentic glomerulonephritis: A clinical and histomorphological analysis of 46 cases. Indian J Pathol Microbiol. 2011;54:497-500.
- [Google Scholar]
- Microscopic polyangiitis (microscopic polyarteritis) Semin Diagn Pathol. 2001;18:3-13.
- [Google Scholar]
- A 25-year experience with pediatric anti-glomerular basement membrane disease. Pediatr Nephrol. 2011;26:85-91.
- [Google Scholar]
- Characteristics and prognosis of Chinese patients with anti-glomerular basement membrane disease. Nephron Clin Pract. 2005;99:c49-55.
- [Google Scholar]
- Anti-glomerular basement membrane (GBM)-antibody-mediated disease with normal renal function. Nephrol Dial Transplant. 1998;13:935-9.
- [Google Scholar]
- Predictors of renal and patient outcomes in anti-GBM disease: Clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30:814-21.
- [Google Scholar]
- Characteristics and outcome of crescentic glomerulonephritis in patients with both antineutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. Clin Rheumatol. 2013;32:1317-22.
- [Google Scholar]
- Goodpasture's disease: A report of ten cases and a review of the literature. Autoimmun Rev. 2013;12:1101-8.
- [Google Scholar]
- Anti-glomerular basement membrane disease: Outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine (Baltimore). 2011;90:303-11.
- [Google Scholar]
- Anti-GBM disease: Predictive value of clinical, histological and serological data. Clin Nephrol. 1993;40:249-55.
- [Google Scholar]
- Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013;83:503-10.
- [Google Scholar]







