Translate this page into:
Postpartum Acute Kidney Injury: Experience of a Tertiary Care Center
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pregnancy-related-acute kidney injury (PR-AKI) had decreased from 40% to 20% in 1960 to <10% in recent series, mostly due to meticulous antenatal management. Postpartum-AKI (PP-AKI) resulting from late obstetric complications has become more apparent after improvement in antenatal care and legalization of medical termination of pregnancy. Women with renal injury in peripartum period admitted to our hospital over a period of 2 years (April 2013 to May 2015) were studied. Of 713 patients of AKI admitted, 61 had PR-AKI with an incidence of 4.27%. Out of the 61 patients, 28 had PP-AKI with an incidence of 1.96%. The mean age of patients with PP-AKI was 26.10 ± 4.3 years. Sepsis was the most common cause accounting for 11 (39.28%) cases followed by postpartum hemorrhage (PPH) in 7 (25%) cases. Renal biopsy was done in nine patients, out of whom four were having cortical necrosis. Patients having diffuse cortical necrosis remained dialysis-dependent. High contribution of sepsis and PPH to PP-AKI in our setting makes it an ideal target for rectification. Protocolized peripartum monitoring and standard clinical practices of asepsis will go long way in decreasing the incidence of PP-AKI and maternal morbidity in our valley.
Keywords
Acute kidney injury
postpartum-acute kidney injury
postpartum hemorrhage
Introduction
Acute kidney injury (AKI) is a heterogeneous syndrome defined by a rapid (over hours to days) decline in the glomerular filtration rate (GFR).[1] Pregnancy-related-AKI (PR-AKI) poses a challenge of different level to clinicians taking care of these patients.[2] The incidence and the mortality associated with PR-AKI have decreased in developed countries from 40% to 20% in 1960 to <10% in recent series mostly due to meticulous antenatal management. No case of PR-AKI was observed in 12,000 and 20,000 births, respectively, in two studies reported from Western countries.[345] With legalization of medical termination of pregnancy, rate of septic abortion as the reason of AKI decreased from 33% in 1980–1985 to 6.3% in 1989–1997 in the West.[6] On the other hand, pregnancy is still responsible for 15–20% of AKI in some regions of world.[67] However, the good thing is that PR-AKI is on decline, reportedly from 14.5% in 1987 to 4.3% in 2005.[89]
AKI resulting from late obstetric complications is well described in literature from the developed countries.[1011121314] However, there is sparse data of PP-AKI in India. Recently reported incidence of PP-AKI is 10.55%,[1516] sepsis and hemorrhage being the commonest etiologies. This study is designed to provide incidence, clinical spectrum, and outcomes of AKI in postpartum period.
Subjects and Methods
This retrospective study was conducted in the Department of Nephrology at Sher-i-Kashmir Institute of Medical Sciences Srinagar, Jammu and Kashmir, India. The data of women having developed renal injury in peripartum period, admitted in our department over a period of 2 years (April 2013 to May 2015) were collected and analyzed for history, clinical findings, record of temperature, blood pressure, urine output, hemogram, blood urea, serum creatinine, liver functions, coagulogram, ultrasonography, and 24 h urinary proteins. Data were also analyzed for postpartum day of admission and duration of stay in hospital. Need, frequency, and mode of dialysis were also noted. Renal biopsy records were retrieved. Maternal outcome was noted as follows:
-
Complete recovery in which creatinine dropped to ≤1.4 mg/dl at the time of discharge/or on follow-up of 12 weeks
-
Partial recovery in which creatinine did not return to 1.4 mg/dl or less after 12 weeks of follow-up or those who lost follow-up
-
Dialysis-dependent: Patients who persist to be anuric and needed dialysis continuously at the time of discharge and on follow-up for more than 3 months and patients with biopsy showing diffuse cortical necrosis
-
Death.
Fetal outcome was noted as live birth, neonatal death and intrauterine fetal death (IUD).
AKI was diagnosed when there was a history of sudden onset oliguria (urine output <400 ml over 6 h) or anuria, with an increase in serum creatinine of more than 0.3 mg/dl/day or rise of or more than 1.5 times from baseline using KDIGO criteria.[17] Patients with preexisting renal insufficiency, hypertension, diabetes, history of renal stone disease, and small size of kidneys were excluded.
Data analysis was done with SPSS software version 20.0 by IBM. Results were recorded as median standard deviation. Chi-square and Fisher's exact tests were used.
Results
Seven hundred and thirteen patients of AKI were admitted over a period of 2 years from May 2013 to April 2015. Out of 713 patients, 61 patients had PR-AKI with an incidence of 4.27%, out of those 61 patients, 28 patients had AKI around or after child birth (PP-AKI) with an incidence of 1.96%. Postpartum constitutes 45.90% of PR-AKI. The mean age of patients with PP-AKI was 26.10 ± 4.3 years with minimum of 17 years and maximum of 35 years [Table 1]. Multigravida constituted 57% of patients and primigravida were 43%. Of the total patients, 61% delivered vaginally and in 39%, cesarean section was performed.

The most common clinical presentation was oligo-anuria (100%) followed by edema (67%), breathlessness (59%), hypotension (45%), fever (41%), hypertension (34%), and encephalopathy (27%) of patients. Most patients were anemic with mean hemoglobin of 10.3 ± 4.2 g/dl. The patients had a mean creatinine of 4.43 ± 2.8 mg/dl with 64% stage 3 of KDIGO class at the time of referral. Mean duration of stay in hospital was 9.96 ± 4.52 days [Table 1]. Sepsis was the most common cause for AKI accounting for 11 (39.28%) cases. Postpartum hemorrhage (PPH) was the cause for AKI in 7 (25%), preeclampsia in 4 (14%), acute pyelonephritis in 3 (11%), atypical hemolytic uremic syndrome in 1 (4%), secondary glomerular disease, i.e., antineutrophil cytoplasmic antibody (ANCA) related vasculitis in 1 (4%), and nonsteroidal anti-inflammatory drug related in 1 patient (3%). Dialysis-requiring AKI was seen in 13 (46%) patients, out of these 13 patients, 9 (69%) patients received intermittent hemodialysis and 4 (31%) received peritoneal dialysis on an average of forty exchanges. Most of our patients recovered despite having advanced renal injury at onset. Out of 28 patients, 15 (54%) recovered completely [Table 2]. Three (11%) patients died. Two died because of sepsis with multiorgan dysfunction and one patient with ANCA-associated vasculitis died. Renal biopsy was done in nine patients. Pathology on renal biopsy was diffuse cortical necrosis in 4 (14.2%) patients of PP-AKI [Table 3]. Patients having diffuse cortical necrosis remained dialysis-dependent. One patient having Pauci immune crescentic glomerulonephritis on renal biopsy died [Table 4]. Among fetal outcomes, total live births were 26 (92.85%), IUD were 2 (7.15%), and 1 (3.5%) died in neonatal period.



Discussion
PR-AKI is on decline in the developed countries. The incidence of AKI in pregnancy has decreased in the past few decades from 40% to 20% in 1960 to <10% in recent literature.[35] In developing countries including India, PR-AKI is showing a decreasing trend, but is still common in some parts. In India, PR-AKI has decreased from 14.5% in 1987 to 4.3% in 2005.[818] Najar et al. reported an incidence of PR-AKI to be 7.04% in 2007 from Kashmir valley.[19] In our study, the incidence of PR-AKI was 4.26% that is in accordance to the declining trend of PR-AKI as reported from other parts of country. However, Naqvi et al. reported an incidence of 25.62% from Pakistan.[20] This may be due to socioeconomic, geographical, and cultural differences. The incidence of PP-AKI in our study was 28 (1.96%). Najar et al. reported an incidence of 1 (2.5%),[19] Gullipalli et al. reported an incidence of 48 (10.5%) from Andhra Pradesh in 2014.[15] Pahwa et al. reported an incidence of 27 (3.59%) from Madhya Pradesh in 2012.[16] Implementation of reproductive and child health care programs, increased utilization of services from qualified health personals and health-care facilities by people, and liberal transport facilities possibly led to decrease in the occurrences of PR-AKI. Wider availability of antenatal and postnatal care and low threshold for referral to tertiary care centers had great impact in reducing the incidence of PP-AKI.
In this study, sepsis is the most common cause for PP-AKI accounting for 11 (39.28%), followed by PPH in seven (25%) patients. Gullipalli et al. reported puerperal sepsis in 16 (33.33%) and uterine hemorrhage including post- and ante-partum hemorrhage as the causes for AKI in 18.88% of patients as causes contributing to PP-AKI in their study. Pahwa et al. reported sepsis in 70.3% and PPH in 22.2% patients of PP-AKI in their study. Naqvi et al. reported sepsis as the cause of PR-AKI in 24% and PPH in 28.03% of cases [Table 5].
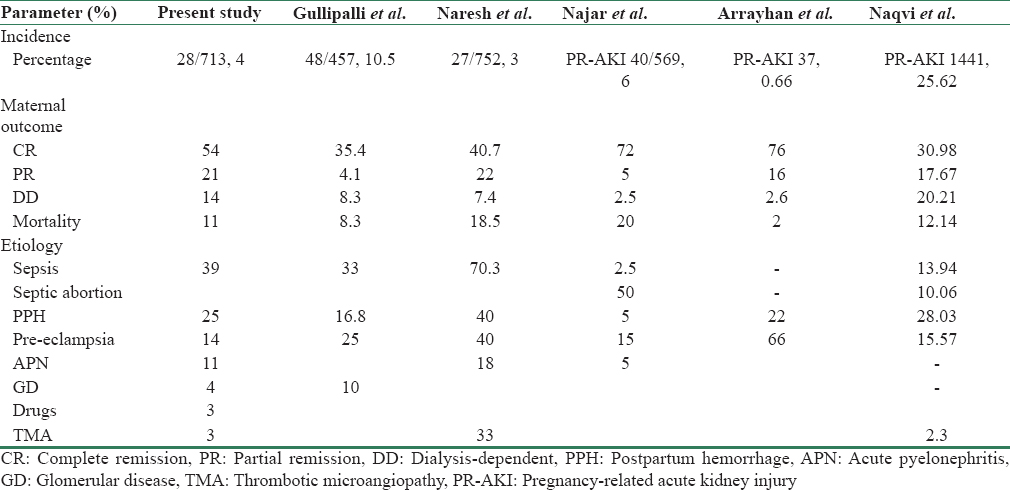
In this study, pre-eclampsia was found in four patients (14%), Gullipalli et al. reported pre-eclampsia/eclampsia as a cause of AKI in [15] 25% of the patients, Pahwa et al.[16] found pre-eclampsia/eclampsia in 40.7% of the patients. We have only 14% patients with pre-eclampsia-associated AKI, possibly we focused more on patients having developed AKI in postpartum period.
We found acute pyelonephritis in three (11%) patients, Najar et al. found acute pyelonephritis in two (5%) patients. Prakash et al. observed urinary tract infection in 9% of pregnancies in their study with maximum occurring in late pregnancy [21] as noted by us.
We found atypical hemolytic uremic syndrome (HUS) in one patient (4%), Pahwa et al. found it in 3% of the patients. Secondary glomerular disease, i.e., ANCA-related vasculitis in one patient (4%) and unexplained in one patient (3%). Gullipalli et al. reported glomerular diseases in 5 (10.41%) patients and unexplained in 2 patients (4.6%).
Dialysis was required in 13 (46%) patients. Out of 28 patients, 15 (54%) recovered completely, 6 (21%) recovered partially, four (14%) patients were dialysis-dependent, and three (11%) patients died. Renal biopsy was done in nine (32.14%) patients. On biopsy, patients having diffuse cortical necrosis – 4 (14.28% of total) remained dialysis-dependent. In patients having acute tubular necrosis – 3 (10.71% of total), 1 (33%) had complete recovery and 2 (66%) have partial recovery. One patient had thrombotic microangiopathy and had partial recovery. One patient having Pauci immune crescentic glomerulonephritis on renal biopsy died. Maternal mortality was observed in three patients (10.71%). Two died because of sepsis with multi-organ dysfunction and one patient with ANCA-associated vasculitis on biopsy. Total live births were 26 (92.85%). IUD was two (7.15%) and one (3.5%) died in neonatal period. Goplani et al. have reported that 54.28% and 12.85% patients had complete and partial recovery of renal function, respectively.[22] In a study by Kumar et al., complete recovery was observed in 51.22% and partial, that is, dialysis independent, in 9.76% patients.[23] Gullipalli P et al. reported the need of dialysis in 47% of patients in their study.[15] The need for dialysis was reported in 70–100% of cases in other series as well.[1019] Overall, maternal mortality was 8.3% in our study. Previously, mortality was very high (55.3%) due to poor antenatal care, late referral, frequent sepsis, and high incidence of bilateral diffuse cortical necrosis.[24] Reported mortality from other studies varies from 23% to 33%.[1125] Sepsis and coagulation abnormalities were the main factors responsible for mortality in the study by Naqvi et al.[7] Kashmir being small valley with accessibility to tertiary care center within short span of time may have contributed to lesser mortality than other studies in addition to round-the-clock availability of specialized care.
Conclusion
In the present study, the incidence of PP-AKI was 1.96%. Sepsis and PPH are the most common causes of AKI, making them ideal targets for intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pathophysiology and etiology of acute kidney injury. In: Jurgen F, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology (4th ed). New York: Elsevier; 2010.
- [Google Scholar]
- Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin Obstet Gynaecol. 1987;1:769-87.
- [Google Scholar]
- Is pregnancy-related acute renal failure a disappearing clinical entity? Ren Fail. 1996;18:575-84.
- [Google Scholar]
- Acute renal failure. In: Brenner BM, ed. The Kidney (7th ed). Philadelphia, PA: Saunders; 2000. p. :1215-70.
- [Google Scholar]
- Changes in frequency and etiology of acute renal failure in pregnancy (1980-1997) Ren Fail. 1998;20:513-7.
- [Google Scholar]
- Acute renal failure of obstetrical origin during 1994 at one center. Ren Fail. 1996;18:681-3.
- [Google Scholar]
- Etio-pathogenesis of acute renal failure in the tropics. Ann Natl Acad Med Sci (India) l. 987;;3:88-99.
- [Google Scholar]
- Acute kidney injury in late pregnancy in developing countries. Ren Fail. 2010;32:309-13.
- [Google Scholar]
- Pregnancy. In: Davidson AM, Cameron JS, Grunfeld JP, eds. Clinical Nephrology (3rd ed). New York, USA: Oxford University Press; 2005. p. :1704-28.
- [Google Scholar]
- Renal cortical necrosis in pregnancy-related acute renal failure. J Indian Med Assoc. 1996;94:227-9.
- [Google Scholar]
- Spectrum of postpartum kidney injury – A tertiary care center experience in a developing nation. IOSR J Dent Med Sci. 2015;14:92-5.
- [Google Scholar]
- Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138.
- [Google Scholar]
- Changing trends in acute renal failure in third-world countries – Chandigarh study. Q J Med. 1989;73:1117-23.
- [Google Scholar]
- Pregnancy related acute kidney injury: A single center experience from the Kashmir Valley. Indian J Nephrol. 2008;18:159-61.
- [Google Scholar]
- Obstetrical acute kidney injury: 25 years' experience from nephrology care unit in Pakistan. Open Access Libr J. 2015;2:e1778.
- [Google Scholar]
- The kidney in pregnancy: A journey of three decades. Indian J Nephrol. 2012;22:159-67.
- [Google Scholar]
- Pregnancy-related acute renal failure: A single-center experience. Indian J Nephrol. 2008;18:17-21.
- [Google Scholar]







