Translate this page into:
Correlation of Pretransplant Donor-specific Antibody Assay Using Luminex Crossmatch with Graft Outcome in Renal Transplant Patients
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The significance of pretransplant anti-human leukocyte antigen antibody levels that are detectable by more sensitive platforms (including the Luminex platform) yet undetected by complement-dependent cytotoxicity (CDC) assay remains unclear. The aim of this study was to determine the clinical significance of the donor-specific antibody (DSA) assay Luminex crossmatch and its impact on short-term renal graft outcome such as acute rejections, graft survival, and graft function. The results of pretransplant DSA-lymphocyte crossmatching (LCXM) assay in 126 renal allograft recipients whose CDCs crossmatches were negative were retrospectively analyzed for correlation with posttransplant outcomes. Of the 126 recipients, 32 (25.4%) had pretransplant DSA positive. Statistically significant association was found between DSA-LCXM positivity with 14th day estimated glomerular filtration rate (eGFR) (P = 0.05), DSA Class I with 3rd (P = 0.014) and 6th month (P = 0.02) eGFR, DSA Class II with 14th day (P = 0.06) and 1st month (P = 0.10) eGFR, mean fluorescent intensity (MFI) DSA with 7th day (P = 0.08) and 14th day (P = 0.09) eGFR, and maximum MFI DSA with 7th day eGFR (P = 0.09). The posttransplant eGFR was higher at various time intervals in DSA-LCXM-negative patients as compared to DSA-positive patients. However, pretransplant DSA-LCXM results did not predict the rejection episodes, graft loss, and 1-year posttransplant 24 h urine protein. Pretransplant DSA detected by LCXM in patients with a negative CDC does not predict adverse short-term outcomes. However, the difference in posttransplant eGFR supports further investigation in long-term effects.
Keywords
Complement-dependent cytotoxicity
donor-specific antibody
Luminex cross match
mean fluorescence intensity
renal transplantation
Introduction
Chronic allograft dysfunction contributed by humoral immune response continues to be a challenge to renal transplant despite effective immunosuppressive regimens.[1] Formation of de novo donor-specific antibody (DSA) against foreign graft human leukocyte antigen (HLA), after renal transplantation, is strongly associated with antibody-mediated graft failure.[23] However, the significance of preformed DSA in a candidate awaiting transplant is still not satisfactorily elucidated. While the complement-dependent cytotoxicity (CDC) assay screens for these antibodies and predicts hyperacute rejection, the significance of antibody levels that are only detectable by the more sensitive platforms including the Luminex platform remains unclear. The primary aim of this study was to determine the impact of pretransplant DSA, detected by Luminex crossmatch – lymphocyte crossmatching (LCXM) (using donor lysate on the Luminex platform) but not on CDC, on short-term graft outcome in terms of acute rejections, grafts survival, and graft function (estimated glomerular filtration rate [eGFR] and proteinuria).
Materials and Methods
Participants included patients who underwent a pre transplant DSA screening by the Luminex crossmatch (Luminex DSA test using donor lysate) (LIFECODES DSA Kit; Gen-Probe, Stamford, CT, USA), on the final pretransplant serum and subsequently received a living-related renal transplant from the same donor over a period of 4 years. Patients with cadaveric transplants and ABO-incompatible transplants were excluded from the study. None of the study participants had desensitization protocol treatment before transplant. All transplanted patients had a negative CDC crossmatch or showed only IgM antibody on the same serum.
CDC detects DSA directed against HLA molecules.[4] It is performed by incubating T- and B-lymphocytes of the donor with serum from the recipient in a multiwell plate with subsequent addition of complement. Complement is activated when recipient antibodies bind to the donor cells. Antibody binding is indicated by a dye, which stains the cells with permeable membranes. The percentage of stained cells is determined using fluorescence microscopy.
Luminex crossmatch involves coating the fluorescent Luminex beads with antibodies specific for Class I or Class II HLA. When treated with donor lysate preparation, these beads will capture the respective HLA antigens of the donor and serve as an HLA target for the detection of DSAs. The presence of specific HLA antibody is indicated by detecting signal from phycoerythrin-bound second anti-human IgG antibody. T- and B-lymphocytes are not separated, but instead unseparated peripheral blood lymphocytes are used to prepare the donor lysate. Instead, the assay uses beads that are coated with antibodies directed at the invariant portion of Class I and Class II antigens, enabling these antigens to be picked up separately from the donor cell lysate. Since the beads used to pick up Class I and Class II antigens are different and are distinctly defined by their intrinsic fluorescent signature, it is possible to clearly distinguish Class I positivity and Class II positivity. Mean fluorescent intensity (MFI) readings above 1000 were taken as positive.
Quality control
All controls were verified to be within set limits prior to validating the test. The background fluorescence is measured with the help of three control beads in the bead mixture. If MFI values of any of these are shown more than 300, then the test is considered invalid. Binding of antihuman globulin fluorescein isothiocyanate conjugate is tested by including a positive control bead coated with IgG. For validation of test results, a minimum MFI of 10,000 is required.
Relevant clinical and laboratory data of the recipients were collected from computerized records. Donors' and recipients' demographic and clinical profile including age, gender, height, weight, nature of donor (living or cadaveric), nature of transplant (preemptive or not), relationship of donor to recipient (categorizing husband to wife and children to mother transplants as a separate entity), total degree of HLA antigen matching as well as for HLA-A, B, DR and DQ were collected. Details of pretransplant DSA using donor lysate test results were collected in the following formats: positivity or negativity, MFI values of Class I and Class II antibodies, a mean of these two MFIs, and a maximum of these two MFIs. MFI values <1000 were considered negative and more than 1000 were considered positive.
The primary outcome of interest was graft survival. The other outcomes studied were graft loss including graft failure and death with functioning graft, biopsy-proven acute rejection, and clinical rejection. All patients with suspected rejection episodes had renal allograft biopsy performed on them. Renal allograft histopathology details during each episode (cellular or vascular rejection) and any histological evidence of chronic rejection (CR) were also studied. The biopsies were graded using Banff '07 classification. The graft outcome was evaluated in each of the following categories: (1) all rejections - either acute cellular rejection (ACR) or acute vascular rejection (AVR) or CR, (2) all biopsy-proven rejections - including either ACR or AVR, (3) AVR alone, (4) graft loss, (5) posttransplant eGFR taken at 7th day, 14th day, 1st month, 3rd month, 6th month, and 1st year was calculated using Cockcroft–Gault formula, (6) 1-year posttransplant eGFR <45 ml/24 h, (7) 1-year posttransplant 24 h urine protein >300 mg, (8) 1-year posttransplant 24 h urine protein, and (9) graft dysfunction at 1 year defined as 1-year posttransplant eGFR <45 ml/24 h or 24 h urine protein >500 mg.
We compared pretransplant DSA-LCXM results, pretransplant Class I and Class II DSA, maximum MFI value of the Class I and Class II DSA, mean MFI value of the Class I and Class II DSA, and demographic characteristics of the study population with the above-mentioned graft outcomes.
All data were analyzed using SPSS Inc. (1999). SPSS Base 11.0 for Windows User's Guide. SPSS Inc., Chicago IL. for the categorical values by Chi-square and Fisher's exact test, continuous variables by independent t-test, and grouped variables analyzed by one-way ANOVA. Correlation at 10% was considered as significant in the univariate analysis. Institutional Review Board approval was obtained.
Results
Baseline characteristics of the study population
One hundred and twenty-six recipients were enrolled in the study. The baseline characteristics are shown in Table 1. There was a correlation between Class II DSA with female gender (P = 0.04), as well as Class II DSA MFI and maximum MFI with total antigen matching (P = 0.043 and 0.05, respectively).

Out of the 126 recipients, 119 (94.4%) had a living donor and 7 (5.6%) had a cadaveric donor. The baseline characteristics of donors are shown in Table 2. Age of the donor correlated with some of the graft outcomes such as all rejection episodes (ACR or AVR or CR) (P = 0.05), 1 year posttransplant GFR <45 ml/24 h (P = 0.01), and 1 year posttransplant GFR (as continuous variable) (P = 0.007).
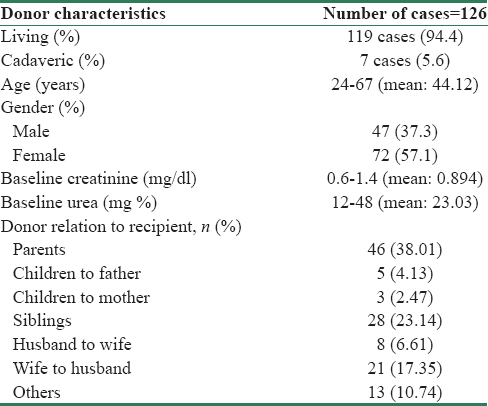
The degree of antigen matching between donor and recipient is shown in Table 3. Majority of them, 83 (69.73%) had 3/8–5/8 antigen matching. Total antigen matching (P = 0.05) had a correlation with the outcome of 1 year posttransplant, 24 h urine protein >300 mg.
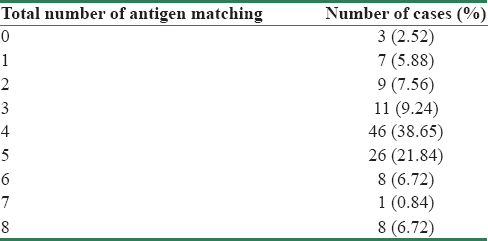
Results of pretransplant donor-specific antibody assay
Of the 126 recipients, 32 (25.4%) showed pretransplant DSA positivity. The antibodies identified were anti-Class I HLA DSA- LCXM in 6 (18.8%), anti-Class II HLA DSA-LCXM in 21 (65.6%), and five (15.6%) recipients had both Class I and Class II DSA-LCXM.
There was no significant correlation between pretransplant DSA-LCXM results, either categorized, quantitative MFI values for Class I or Class II, maximum MFI or mean (of Class I and Class II) MFI with rejection episodes [Table 4]. There was, however, some association between DSA-LCXM positivity with 14th day eGFR (P = 0.05), DSA Class I with 3rd (P = 0.014) and 6th month (P = 0.02) eGFR, DSA Class II with 14th day (P = 0.06) and 1st month (P = 0.10) eGFR, MFI DSA with 7th day (P = 0.08) and 14th day (P = 0.09) eGFR, and maximum MFI DSA with 7th day eGFR (P = 0.09) [Table 5].


The estimated GFR of the recipients at posttransplant 7th and 14th days, 1st, 3rd, 6th months, and 1st year in DSA-negative patients remained higher than DSA-positive patients [Figure 1]. Moreover, the difference of eGFR between the two groups was found to progressively increase with time (mean difference of GFR of 7.6 at 3rd month [P = 0.01] and 7.7 at 6th month [P = 0.02].
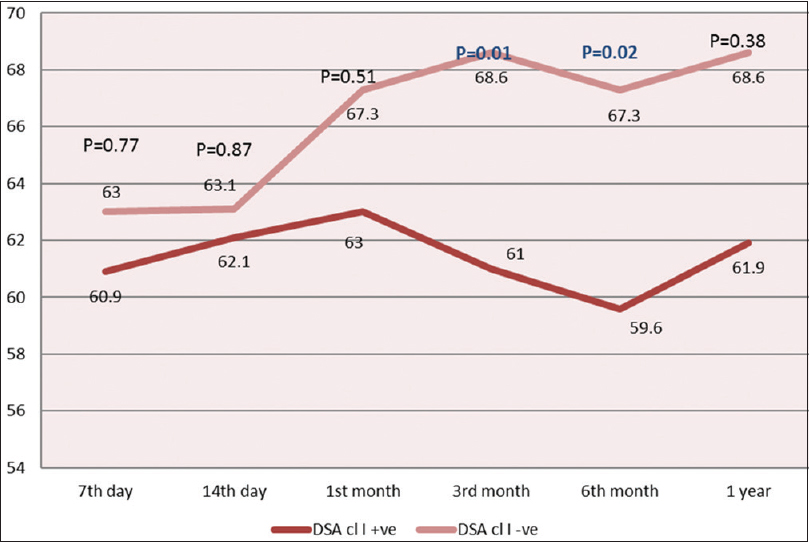
- Graph of posttransplant glomerular filtration rate (ml/min) at various time intervals in donor-specific antibody-positive and donor-specific antibody-negative patients. DSA cl I: Donor-specific antibody Class I; DSA cl II: Donor-specific antibody Class II
Discussion
The correlation between total antigen matching as well as the age of the donor with adverse graft outcomes supports previous studies.[56]
Exposure to donor antigens during pregnancy could explain the correlation between Class II DSA with female gender in our study. The correlation between Class II DSA MFI and maximum MFI with the total antigen matching implied an association between sensitization and donor recipient antigen mismatching in our study population. However, the clinical implication of these associations is difficult to assess without further details on the nature of sensitizing events and the relationship between the recipient and the donor, which could account for sensitization originating from the latter.
Our present study results showed no significant difference in allograft outcome between pretransplant DSA-positive and pretransplant DSA-negative groups and this is in agreement with our previous study[7] on a small number of recipients with a short-term follow-up and with studies published from other centers, such as by Phelan et al.,[8] Van den Berg-Loonen et al.,[9] and Süsal et al.[10]
However, some studies have reported contrasting results, which indicate that pretransplant DSA and its class specificity have significant impact on acute rejection rates and graft survival. Some of these were conducted on recipients who were positive on CDC at the time of transplant.[1112] In contrast, in our study, all the recipients had a negative immediate pretransplant CDC crossmatch.
In view of the correlation of DSA with posttransplant eGFR in our study, and considering that this is considered a surrogate marker for long-term outcome, it may be reasonable to presume that pretransplant DSA may have a significant effect on the long term.[13]
A few studies[1415] report the minimal impact of persistent low-level pretransplant DSA on early graft outcome in presensitized recipients who have undergone pretransplant desensitization. The predominant immunosuppressive regimen used is (for low immunologic risk individuals defined as living donors with crossmatch negative by CDC) basiliximab induction with prednisone, tacrolimus and mycophenolate mofetil. Anti Thymocyte Globulin was used as an induction agent among living donor transplant recipients only in patients with historical crossmatch positivity during this study period. The clinicians were clearly blinded to the DSA results at the time of the study, thereby avoiding any potential bias and changes in immunosuppression used. No other desensitization protocol was used among the study patients.
Our study is one of the few studies using Luminex crossmatch (DSA testing based on donor lysate-derived HLA-coated beads) for detecting pretransplant DSA, which uses antigen extracted from donor lysates, thereby permitting us to perform a real donor–recipient crossmatch on the Luminex platform. The assay indeed does give false-positive and false-negative results on occasion. False-positive results are more frequent with Class II and are relatively infrequent with Class I. False-negative results occur because of the admitted insensitivity toward pick up of antibodies to HLA DQ and DP. Arguably, the relevance of antibodies toward these loci is only recently established and many centers do not yet have algorithms that incorporate detection and management of these, or even typing the donor at these loci.
The long-term impact of pretransplant DSA is yet to be conclusively assessed, since it might take years for the HLA-DSA to cause a detrimental clinical effect. Additional allograft biopsies are needed to exclude late subclinical or chronic antibody-mediated rejection.[16]
However, it is our view that the decision of denying transplantation is neither ethical nor economical if allosensitization proves to be clinically manageable. In our study, if we had considered the mere positivity of pretransplant DSA alone (with a negative final CDC crossmatch) as an absolute contraindication for transplantation, 24% (30/126) of these patients would not have received a renal transplant.
Limitations of the study
In our study, the maximum MFI value of Class I DSA is 2818 and that for Class II DSA is 4184. Hence, the significance of higher MFI values could not be studied. In the presence of negative pretransplant CDC crossmatch and isolated positive pretransplant DSA, factors such as titer of the antibody, subclass of the immunoglobulin and its complement-fixing capacity have been shown to play a role in deciding which antibodies detected by Luminex are clinically significant.[17] Our study does not examine these aspects.
The cutoff of MFI values <1000 as negative and more than 1000 as positive used in our study is an arbitrary value. The use of flat cutoffs above 1000 for the raw MFI is simple and was modified from other published studies.[18] The results from this method are not unduly distorted by background control values, provided these are within acceptable limits (<300). Our approach was validated using samples from the flow cytometric crossmatch program of the UK NEQAS and correlates well with our own flow cytometric crossmatch results. C4d, which is a major predictor of antibody-mediated graft rejection, was not done in all the suspected cases due to technical reasons.
Conclusion
Our study suggests that pretransplant DSA detected exclusively on the Luminex platform with a negative CDC should not be construed as a contraindication to transplantation. Further studies are needed to determine long-term effects and the influence of higher MFI values.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378-83.
- [Google Scholar]
- Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408-15.
- [Google Scholar]
- De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 2012;94:172-7.
- [Google Scholar]
- Comparison of the established standard complement-dependent cytotoxicity and flow cytometric crossmatch assays with a novel ELISA-based HLA crossmatch procedure. Histol Histopathol. 2006;21:1115-24.
- [Google Scholar]
- Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70:306-10.
- [Google Scholar]
- 008 pre transplant anti HLA antibodies and donor specific antibodies detected on the Luminex platform – Impact on renal graft outcome. Indian J Transpl. 2011;5:12.
- [Google Scholar]
- Living donor renal transplantation in the presence of donor-specific human leukocyte antigen antibody detected by solid-phase assay. Hum Immunol. 2009;70:584-8.
- [Google Scholar]
- Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation. 2008;85:1086-90.
- [Google Scholar]
- No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: A Collaborative Transplant Study report. Transplantation. 2011;91:883-7.
- [Google Scholar]
- Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582-9.
- [Google Scholar]
- Clinical relevance of anti-HLA donor-specific antibodies detected by Luminex assay in the development of rejection after renal transplantation. Transplantation. 2012;27(94):338-44.
- [Google Scholar]
- Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol. 2015;26:20-9.
- [Google Scholar]
- Persistence of low levels of alloantibody after desensitization in crossmatch-positive living-donor kidney transplantation. Transplantation. 2004;78:221-7.
- [Google Scholar]
- Blood levels of donor-specific human leukocyte antigen antibodies after renal transplantation: Resolution of rejection in the presence of circulating donor-specific antibody. Transplantation. 2007;84:876-84.
- [Google Scholar]
- Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046-56.
- [Google Scholar]
- C4d fixing, luminex binding antibodies – A new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809-15.
- [Google Scholar]







