Translate this page into:
Hemostatic Abnormalities in Severe Renal Failure: Do They Bark or Bite?
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abnormal primary hemostasis is believed to be the most significant contributor to uremic bleeding. This study aimed to describe the prevalence and profile of primary and secondary hemostatic disorders in patients with chronic kidney disease (CKD) Stages 4 and 5 and to determine their association if any, with degree of uremia. Stages 4 and 5 predialysis CKD patients attending nephrology outpatient clinic were prospectively recruited and the following bleeding parameters were measured in all patients: platelet count, bleeding time (BT), Factor VIII assay, von Willebrand factor antigen (vWF:Ag), vWF:ristocetin cofactor activity (vWF:RCo), ratio of vWF:ristocetin cofactor activity to vWF antigen (vWF:RCo/vWF:Ag), prothrombin time (PT), and activated partial thromboplastin time (aPTT). Forty-five patients (80%, males) with a mean age of 39.4 years, 82% (n = 37) in Stage 5 CKD, were recruited for the study. The prevalence of thrombocytopenia was significantly higher among patients from West Bengal (15/26, 57.7%) compared to other study patients (2/19, 10.5%; P = 0.001); however, all had macrothrombocytes with normal BT, suggestive of the Harris syndrome. Factor VIII, vWF:Ag, vWF:RCo, vWF:RCo/vWF:Ag ratio, BT, PT, and aPTT were abnormal in 0 (0%), 0 (0%), 0 (0%), 4 (8.8%), 1 (2.2%), 7 (15.6%), and 5 (11.1%) patients, respectively. Except for thrombocytopenia, the prevalence of hemostatic abnormalities did not differ between CKD Stages 4 and 5. Hemostatic abnormalities are uncommon in Stages 4–5 CKD and except for thrombocytopenia, are not associated with degree of uremia. Constitutional macrothrombocytopenia is associated with normal BT even in CKD.
Keywords
Bleeding time
chronic kidney disease
Harris syndrome
hemostasis
macrothrombocytopenia
Introduction
Uremic bleeding is multifactorial. Various abnormalities in platelet–platelet and platelet–vessel wall interactions have been described; however, abnormal primary hemostasis (vasoconstriction, platelet adhesion, and aggregation) is believed to have a much greater role in its pathogenesis than defects in secondary hemostasis (coagulation) or fibrinolysis.[1] While there are data on the prevalence of coagulation abnormalities in Indian chronic kidney disease (CKD) patients,[2] primary hemostatic abnormalities have not previously been studied. This study aimed to describe the prevalence of primary and secondary hemostatic abnormalities in predialysis patients with Stages 4 and 5 CKD at a tertiary center in South India.
Materials and Methods
All patients with confirmed Stages 4 and 5 CKD based on clinical, laboratory, ultrasonographic, and, where available, histopathological reports, who attended the Nephrology Outpatient Department of our institution over a 5-month period from September 2006 to January 2007 and consented to participate in the study, were included in the study. We excluded patients with a history of bleeding episodes in childhood, those receiving erythropoietin, anticoagulation, antiplatelet agents or antibiotics, those with an ongoing or recent (<2 weeks) infection, those with abnormal liver function tests or known chronic liver disease, and those who had initiated dialysis. Informed consent was taken in the prescribed format from all study participants, and in the case of minors, from their parents. Apart from blood urea, serum creatinine, hemoglobin, 24 h urine protein, and serum albumin, the following tests for primary hemostasis were performed on all patients: platelet count by coulter method (normal range: 1.5–4.5 × 105/mm3), bleeding time (BT) by Modified Ivy's method (normal range: 2–6 min) performed by a single technician, Factor VIII assay by a one-stage clot-based assay using the CA 1500 system (normal range: 50–150%), von Willebrand factor antigen (vWF:Ag) using the immunoturbidimetric method on the automated ACL-10000 system (normal range: 50–175 U/dl), and vWF:ristocetin Cofactor activity (vWF:RCo) by the agglutination method using the nephelometric channel of the ACL-10000 system (normal range: 50%–175%). All patients also underwent basic evaluation for disorders of secondary hemostasis with prothrombin time (PT, normal range: 10.3–13.4 s) and activated partial thromboplastin time (aPTT, normal range: 28–36.2 s) on the ACL advanced automated system. In patients with thrombocytopenia by coulter method, a peripheral blood smear for platelet morphology was performed. Estimated glomerular filtration rate was calculated using the 4-variable abbreviated MDRD equation. The study was approved by the Institutional Review Board and Ethics Committee of our institution.
The frequency of hemostatic abnormalities was expressed in percentage. Quantitative variables were expressed as mean ± standard deviation for normally distributed and median with range for skewed data. For continuous variables, means were compared using Student's t-test for normally distributed and Mann–Whitney U-test for skewed data. Significance was determined by Chi-square test, with the Yates continuity correction factor being used where at least one cell of the 2 × 2 table had an expected count <5.
Results
A total of 45 patients who met inclusion criteria and consented to take part in the study were included in the study. Of these, 36 (80%) patients were male. The mean age of the study participants was 39.4 years (range: 13–66 years). Of the 45 study participants, 37 (82.2%) had Stage 5 chronic kidney disease. The majority of patients (46.6%) had unknown native kidney disease [Table 1]. The mean hemoglobin was 8.4 g/dl with only 9 patients (20%) having a hemoglobin of 10 g/dl or above [Table 2].

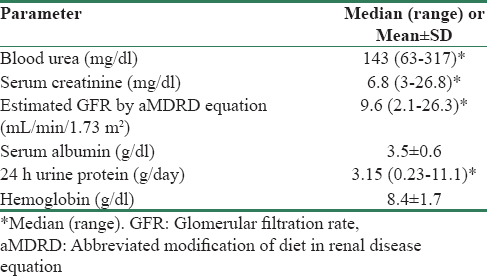
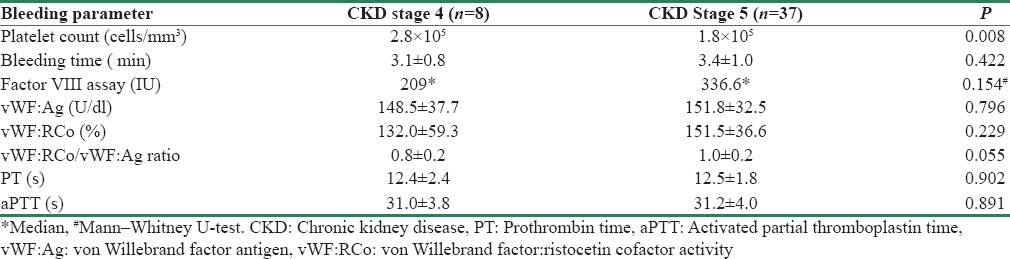
Table 3 gives the results of tests for hemostasis performed on study participants.
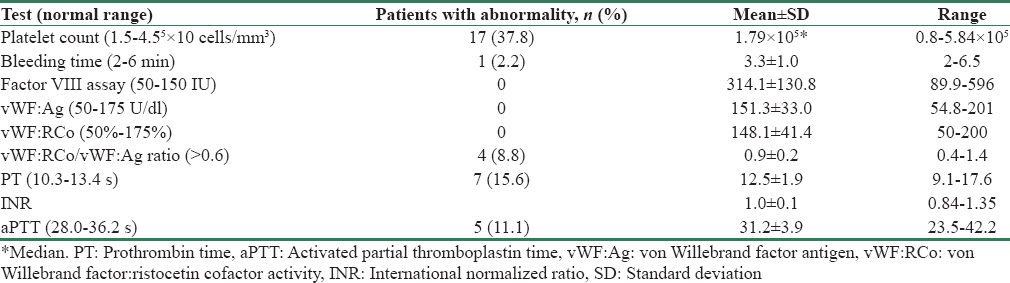
Seventeen patients (37.8%) had a platelet count <1.5 × 105 cells/mm3; however, only 1 patient had a platelet count <1 × 105 cells/mm3. As shown in Figure 1, a large proportion (n = 26, 57%) of our study population hailed from West Bengal, and this group had a much higher prevalence of thrombocytopenia compared to the other study patients (15/26, 57.7% vs. 2/19, 10.5%, P = 0.001). However, all patients with thrombocytopenia had macrothrombocytes on peripheral smear and all but one had a normal BT, suggestive of constitutional macrothrombocytopenia or the Harris syndrome.
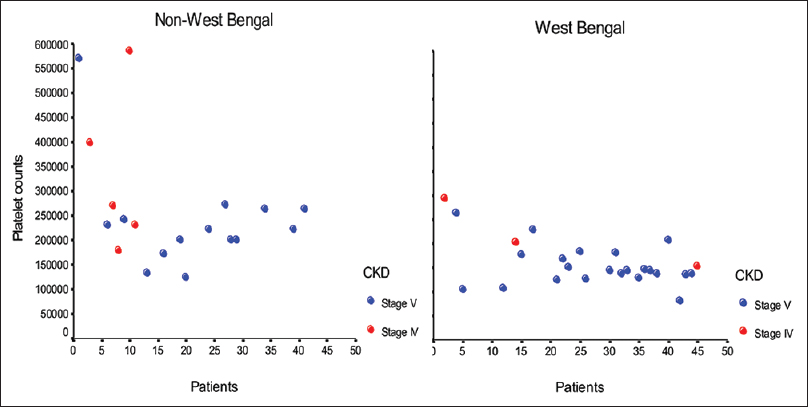
- Distribution of platelet count in West Bengal and non-West Bengal study participants (expressed per mm3)
No patient had low Factor VIII, vWF:Ag, or vWF:RCo. On the contrary, Factor VIII levels were elevated in 88.8% of patients. Four patients (8.8%) had an abnormal vWF:RCo/vWF:Ag ratio despite having normal values for vWF:RCo and vWF:Ag, suggesting a functional vWF abnormality.
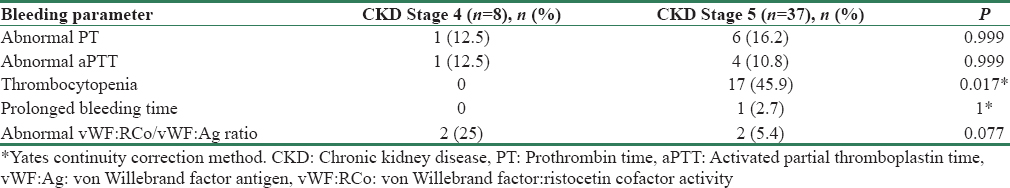
The number of patients with abnormal (above the normal range) PT and aPTT were 7 (15.6%) and 5 (11.1%), respectively. In all patients, the abnormality was corrected with addition of pooled normal plasma, indicating a factor deficiency. Table 4 gives the prevalence of isolated and combined hemostatic abnormalities and their likely inference. Figure 2a-g graphically represents the distribution of test results in the study population.
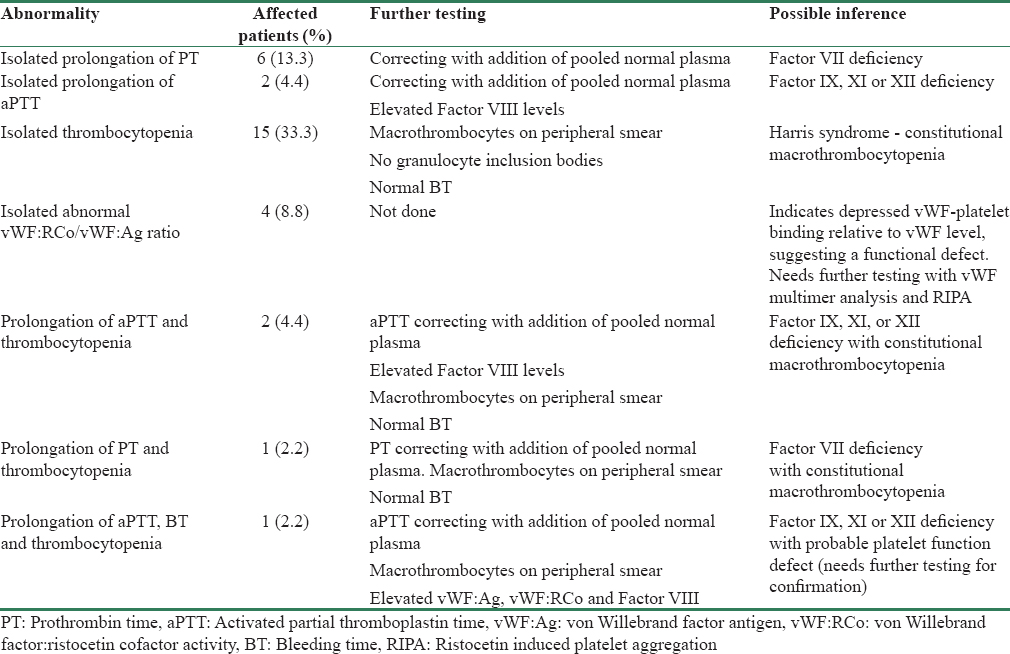

- Distribution of hemostatic test abnormalities in the study population. (a) Platelet count expressed per mm3 (horizontal bars represent normal range of values). (b) Bleeding time in minutes (horizontal bars represent normal range of values). (c) Factor VIII concentration in IU (horizontal bars represent normal range of values). (d) von Willebrand Antigen concentration in U/dl (horizontal bars represent normal range of values). (e) Ristocetin cofactor activity in % (horizontal bars represent normal range of values). (f) Activated partial thromboplastin time in seconds (horizontal bars represent normal range of values). (g) Prothrombin time in seconds (horizontal bars represent normal range of values)
Only one patient had an abnormal BT (6.5 s), and this patient also had macrothrombocytopenia (1.4 × 105 cells/mm3) and an abnormal aPTT (42.2 s), which corrected with pooled normal plasma. vWF:Ag, vWF:RCo, and Factor VIII levels were elevated, ruling out von Willebrand disease. This picture was suggestive of a coagulation factor defect involving the intrinsic pathway and a possible platelet function defect, though further tests are needed for confirmation, which were outside the scope of the study.
The mean platelet count was lower in CKD Stage 5 (P = 0.008). Apart from thrombocytopenia, there was no association between hemostatic abnormalities and degree of uremia [Tables 5 and 6].
Discussion
More than half of Indian CKD patients present to a nephrologist in CKD Stage 5,[3] making them the ideal population to study the prevalence and severity of uremic hemostatic abnormalities. In keeping with data from the CKD registry of India, our study population was predominantly male and presented in CKD Stage 5, but they were younger than the average CKD patients described in the registry. This may be related to the high prevalence of CKD of unknown etiology in this group, an entity that is associated with younger age at presentation.[4]
Various defects in primary hemostasis have been described in uremia. These include abnormalities in platelet number, dense granule content, concentration of intracellular ADP, serotonin and cyclic AMP, release of platelet α granules, calcium ion mobilization, arachidonic acid metabolism, cyclooxygenase activity, GPIIbIIIa binding, platelet aggregation and adhesion, vWF activity, prostaglandin I2 and nitric oxide production by the vessel wall, and altered blood rheology due to anemia.[1]
Hemostatic abnormalities
Platelet count
Platelet count in uremia has been found to be decreased in 16%–50%; however, thrombocytopenia is usually mild and rarely below 80,000 cells/mm3.[1] Platelet volume has also been reported to be low, a factor contributing to prolonged BT.[5] Our study population had a high prevalence of thrombocytopenia (17/45, 37.8%); however, all had macrothrombocytes on peripheral smear. This constitutional macrothrombocytopenia, also called the Harris platelet syndrome, was first described in blood donors from West Bengal[6] and is autosomal-dominant inherited.[7] In West Bengal, Nepal, Bhutan, and Bangladesh, the population incidence of this platelet abnormality exceeds 30%[8] but is much less common in North India.[9] It is distinguished from other hereditary macrothrombocytopenias such as Bernard–Soulier syndrome and MYH9-related disease by the absence of childhood bleeding episodes and granulocyte inclusion bodies on peripheral smear, normal platelet aggregation, and normal BT,[7] as was the case in our patients. This condition is important to recognize in order to avoid unnecessary investigations and inappropriate treatment.
von Willebrand factor antigen
vWF is a multimeric protein that mediates platelet adhesion to the injured vessel and is released from endothelial cells and megakaryocytes. Its deficiency, as seen in von Willebrand disease Type 1, is associated with prolonged BT and a bleeding diathesis.[1011] None of our patients had low vWF:Ag concentration, and 10 patients (22.2%) had values above the normal range. Other authors have also found the concentration of vWF:Ag to be normal[1213] or elevated[1415] in renal failure. The structure of vWF multimers has also been shown to be normal in renal failure.[16]
von Willebrand factor: ristocetin cofactor activity
vWF:RCo is a functional assay that measures the ability of vWF to bind to platelets. A value below the normal range, as seen in von Willebrand disease Type 2, results in a bleeding diathesis even if vWF:Ag concentration is normal. The positively charged antibiotic ristocetin causes a conformational change in platelet GP1b receptor, promoting vWF binding, resulting in platelet agglutination. None of our patients had low vWF:RCo activity and 12 patients (26.6%) had values above the normal range. Other investigators have also found vWF:RCo to be elevated in uremia.[1516]
von Willebrand factor: ristocetin cofactor activity/von Willebrand factor: antigen ratio
The ratio of vWF:RCo/vWF:Ag indicates the efficiency of vWF platelet binding relative to the vWF concentration; hence, a value <0.6 indicates a vWF function defect even if vWF:RCo and vWF:Ag are in the normal range and results in a bleeding diathesis, as seen in von Willebrand disease Type 2.[17] Four patients had an abnormal ratio, despite having individual values of vWF:RCo and vWF:Ag in the normal range and all had normal BT. None of these had low Factor VIII levels or thrombocytopenia. This finding indicates either a selective deficiency of high molecular weight multimers of vWF, a condition analogous to that seen in von Willebrand disease Type 2A, or a vWF mutation that impairs its binding to the platelet GP1b, analogous to von Willebrand disease Type 2M.[17] A defect in the platelet binding site of vWF has not been demonstrated in CKD; hence, the former is the most likely explanation.[16] Three points need to be noted here. First, vWF function is specifically attributed to larger multimers of vWF[10] and a selective defect in the in vivo release of the largest vWF multimers has been described in CKD.[16] Second, this defect can exist despite normal vWF:Ag concentration, vWF:RCo activity, and BT and requires measurement of the vWF:RCo/vWF:Ag ratio. Third, its management involves administration of cryoprecipitate[13] or deamino-8-D–arginine vasopressin (DDAVP).[18] The former directly supplies, and the latter stimulates release of, large vWF multimers.
Factor VIII assay
Factor VIII is measured as part of the assessment of the functional status of vWF, which binds and stabilizes it in circulation. Its deficiency is associated with hemorrhagic disease as seen in hemophilia A. None of our patients had low Factor VIII activity. On the contrary, we found markedly elevated levels of Factor VIII in 88.8% of our patients. This has been corroborated by other investigators.[1519] Factor VIII and vWF are secreted by endothelial cells and platelets.[20] High Factor VIII levels in uremia are related to high vWF (which increases Factor VIII half-life) and endothelial damage secondary to oxidative stress, uremic chronic inflammatory state, and shear stress[21] since Factor VIII is also an acute phase reactant.[22] The clinical relevance of elevated Factor VIII in uremia lies in its link to accelerated atherosclerosis, arterial and venous thrombosis, which are important causes of morbidity and mortality in CKD.[23]
Bleeding time
BT is influenced by vessel wall contractility, vWF concentration and function, platelet number, and function. It is prolonged in uremia and corrected by dialysis.[24] Apart from abnormalities in the vWF-platelet-vessel wall axis, the critical role of anemia in prolonging BT has been demonstrated.[25] In the presence of adequate hematocrit, red cells occupy the axial center of the blood vessel, pushing platelets to the periphery, thus promoting easy adhesion at the site of vessel injury.[26] In addition, red blood cells release ADP, a powerful platelet aggregator,[27] and hemoglobin has a scavenging effect on nitric oxide, a vasodilator and inhibitor of platelet function whose synthesis is upregulated in renal failure.[28] In uremic patients, BT can be normalized by raising hematocrit to 30%.[29] Although BT is invasive, time consuming, and difficult to standardize, it is the best in vivo test for assessment of primary hemostasis in uremia and correlates better with clinical bleeding than platelet aggregation tests.[30]
It is noteworthy that BT in our study was normal in all but one patient, despite the high prevalence of thrombocytopenia (37.8%) and mean hemoglobin of 8.4 g/dl. There are several explanations for this. First, the degree of thrombocytopenia observed was mild – no patient had a platelet count <80,000 cells/mm3, a level considered adequate for hemostasis.[1] Second, all patients with thrombocytopenia had macrothrombocytopenia, a condition associated with normal BT because giant platelets are distributed away from the axial center, near the vessel wall. Third, it has been suggested that the high concentration of vWF and Factor VIII in uremic plasma is able to compensate for defective platelet adhesion.[31] The high mean vWF:Ag and Factor VIII concentration in our study population supports this theory. Fourth, while the KDIGO guidelines on anemia management in CKD define anemia as hemoglobin level <13 g/dl in males and <12 g/dl in females,[32] this guideline needs to be revisited in the Indian context, where healthy adults have a hemoglobin cutoff value (corresponding to the 5th percentile of population values) at least 1 g/dl lower than their Western counterparts,[33] a difference that is likely to be aggravated in CKD patients.
Prothrombin time
The PT, which is a measure of the integrity of the extrinsic and final common pathways of the coagulation cascade, was abnormal in 7 patients. In all cases, PT corrected with addition of pooled normal plasma, and aPTT was normal, suggesting a factor VII deficiency.[34] Factor VII is synthesized in the liver and its deficiency may be associated with liver disease, Vitamin K deficiency, anticoagulation, sepsis, malignancy, or antiphospholipid syndrome,[34] which had been ruled out in our patients. It is likely that the Factor VII deficiency was congenital and related to genetic polymorphisms, previously described in the Indian population.[35] Clinical manifestations of congenital Factor VII deficiency vary greatly depending on genotype, with up to 47% of heterozygotes being asymptomatic.[36]
Activated partial thromboplastin time
Five patients had a prolonged aPTT, and in all cases, PT was normal and aPTT corrected with addition of pooled normal plasma, suggesting a Factor VIII, IX, XI, or XII deficiency.[34] Since Factor VIII levels were elevated in all these patients, further testing for Factor IX, XI, or XII deficiency was required, which was not done in our patients. Out of these, Factor XII deficiency does not result in increased bleeding risk.[34]
Comparison of hemostatic abnormalities in patients with Stage 4 and 5 CKD
Mean platelet count was lower, and frequency of thrombocytopenia is higher in Stage 5 CKD. CKD is associated with thrombocytopenia due to decreased thrombopoietic activity[37] and the inherited thrombocytopenia of Harris syndrome is therefore exacerbated by CKD.
The frequency of abnormalities in PT and aPTT did not vary between the two groups, since these were most likely due to factor deficiencies that are congenital. The frequency of low vWF:RCo/vWF:Ag ratio was also not different between the groups, indicating a genetic predisposition that is made manifest by CKD.
The bleeding diathesis of uremia – Does it exist?
Gastrointestinal bleeding is more common and has a higher mortality in CKD patients than in the general population.[38] The increased risk for, and severity of, bleeding in these patients has been attributed to the hemostatic abnormalities of CKD, yet our study showed that both primary and secondary hemostasis are largely normal even in advanced kidney disease. How can this be explained?
The most common sites of bleeding in uremic patients are from puncture sites, mucosal and serosal surfaces (epistaxis, gastrointestinal and genitourinary bleeds, hemorrhagic pericarditis and pleuritis), and subdural hematomas.[39] CKD patients have risk factors for injury to all these sites – frequent venepuncture; increased prevalence of peptic ulcers, angiodysplasia, mesenteric ischemia and diverticulosis; glomerular disease, acquired cystic, and polycystic kidney disease; pleural and pericardial inflammation; hypertension and head trauma due to falls.[39] The risk is compounded by age, anticoagulation, antiplatelet agents, and anemia. Likewise, post kidney biopsy bleeds have been linked to hypertension, the gauge of the biopsy needle, depth of needle insertion, number of needle passes, and degree of arteriosclerosis in the biopsy tissue rather than BT.[40] Uremic hemostatic abnormalities, though clinically insignificant in themselves, acquire importance when superimposed on this conglomeration of pathologies. We therefore suggest that greater attention be paid to these risk factors, which are far more likely to cause bleeding in uremic patients than primary or secondary hemostatic abnormalities.
Practical implications for the clinician
Our findings suggest that Factor VIII and vWF (vWF:Ag) concentration and vWF functional activity (vWF:RCo) are preserved in uremia and do not contribute to uremic bleeding. A small proportion of CKD patients (8.8% in this study) have a selective defect in large vWF multimers, resulting in an abnormal vWF:RCo/vWF:Ag ratio, which can be corrected with cryoprecipitate or DDAVP. It is important to note that such patients may also have a normal BT and thus may not be diagnosed before a procedure. Thrombocytopenia in a CKD patient, especially those from West Bengal and the North East, should prompt examination of a peripheral smear to look for giant platelets, which is not associated with a bleeding diathesis provided clotting factor deficiency has been ruled out. Because CKD patients may have inherited clotting factor deficiencies, PT and aPTT should be tested before an invasive procedure and any clinically significant factor deficiency should be managed by administration of the appropriate factor concentrate or fresh frozen plasma. The BT is the best in vivo test to assess bleeding risk in CKD patients, and though cumbersome and requiring a skilled technician, may help to identify the rare patient with a clinically significant platelet function defect (one patient, in this study). While a hematocrit of 30% has been shown to aid in adequate hemostasis in CKD patients, our patients had a normal BT with much lower hemoglobin concentrations, suggesting that invasive procedures may be safe in anemic Indian CKD patients, in the absence of other hemostatic defects.
Our study showed that platelet function, as assessed by BT, is normal in the vast majority of predialysis CKD patients, and therefore, antiplatelet agents can be safely prescribed. Yet, antiplatelet agents have been shown to increase the risk of major bleeding in CKD patients, irrespective of the type of antiplatelet agent used or stage of CKD.[41] This indicates that it is not the degree of uremia or type of antiplatelet agent that is the culprit, but the presence of predisposing factors for major bleeds, such as peptic ulcers and uncontrolled hypertension which nephrologists should screen their patients for, before starting antiplatelet agents. When CKD patients on single or dual antiplatelet agents require emergency invasive procedures, platelet concentrate with or without DDAVP may be administered.[4243]
Limitations
Although our center is situated in Southern India, a large proportion of our patients hail from West Bengal, North East, and Central India. This may account for the high proportion of patients with macrothrombocytopenia, which may not be true of studies from other parts of India. Our study population was small, and it is likely that a higher frequency of disorders of primary hemostasis may have been detected if a larger number were studied. We did not perform platelet function tests such as platelet aggregation in response to ADP, collagen, epinephrine, or platelet function analyzer 100, which may have been more sensitive for the detection of specific platelet function abnormalities. However, the clinical relevance of in vitro tests versus an in vivo test such as the BT, especially in CKD, where there are multiple defects in hemostasis, is debatable.[30] Finally, our study cohort did not undergo any invasive procedure, and so the correlation between the bleeding risk, as determined by the tests performed, and clinical bleeding episodes, could not be determined.
Conclusion
Our study did not demonstrate any major defects of primary hemostasis in predialysis Stages 4 and 5 CKD. Our study population had a high prevalence of constitutional macro thrombocytopenia (Harris syndrome), which was associated with normal BT but exacerbated by CKD. Coagulation factor deficiency was demonstrated in 26.6% but was likely congenital and unrelated to the degree of uremia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We express our gratitude to the technicians of the Clinical Pathology Laboratory of our hospital for their whole hearted cooperation in this study, and Nithya Neelakantan for the statistical analysis.
References
- Pre-tertiary hospital care of patients with chronic kidney disease in India. Indian J Med Res. 2007;126:28-33.
- [Google Scholar]
- What do we know about chronic kidney disease in India:First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- The decreased circulating platelet mass and its relation to bleeding time in chronic renal failure. Thromb Haemost. 1991;65:11-4.
- [Google Scholar]
- Asymptomatic constitutional macrothrombocytopenia among West Bengal blood donors. Am J Med. 2002;112:742-3.
- [Google Scholar]
- Harris platelet syndrome – Underdiagnosed and unrecognized. Arch Pathol Lab Med. 2008;132:1546.
- [Google Scholar]
- Macrothrombocytopenia in north India: Role of automated platelet data in the detection of an under diagnosed entity. Indian J Hematol Blood Transfus. 2015;31:61-7.
- [Google Scholar]
- Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011;9(Suppl 2):s3-8.
- [Google Scholar]
- Bleeding in renal failure: Is von Willebrand factor implicated? Br Med J. 1977;2:359-61.
- [Google Scholar]
- Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980;303:1318-22.
- [Google Scholar]
- Increased factor VIII/von Willebrand factor antigen and von Willebrand factor activity in renal failure. Am J Med. 1979;66:226-8.
- [Google Scholar]
- Plasma and platelet von Willebrand factor defects in uremia. Am J Med. 1988;85:806-10.
- [Google Scholar]
- Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8-12.
- [Google Scholar]
- Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int. 2001;60:1844-50.
- [Google Scholar]
- Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol. 2001;21:731-8.
- [Google Scholar]
- Skin bleeding time for the evaluation of uremic platelet dysfunction and effect of dialysis. Clin Appl Thromb Hemost. 2012;18:185-8.
- [Google Scholar]
- Low haematocrit and prolonged bleeding time in uraemic patients: Effect of red cell transfusions. Br J Haematol. 1985;59:139-48.
- [Google Scholar]
- Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961;192:531-2.
- [Google Scholar]
- Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985;233:679-85.
- [Google Scholar]
- Uraemic bleeding: Role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;2:1013-5.
- [Google Scholar]
- Bleeding time in uremia: A useful test to assess clinical bleeding. Am J Hematol. 1979;7:107-17.
- [Google Scholar]
- High von Willebrand factor concentration compensates a relative adhesion defect in uremic blood. Blood. 1990;75:1498-508.
- [Google Scholar]
- KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335.
- [Google Scholar]
- Establishment of reference intervals in Indian population. Indian J Clin Biochem. 2005;20:110-8.
- [Google Scholar]
- How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864-73.
- [Google Scholar]
- Molecular basis of hereditary factor VII deficiency in India: Five novel mutations including a double missense mutation (Ala191Glu; Trp364Cys) in 11 unrelated patients. Haematologica. 2007;92:1002-3.
- [Google Scholar]
- Factor VII deficiency: Clinical manifestation of 717 subjects from Europe and Latin America with mutations in the factor 7 gene. Haemophilia. 2009;15:267-80.
- [Google Scholar]
- Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron. 1987;45:207-10.
- [Google Scholar]
- Gastric and pancreatic function in patients with end-stage renal disease. J Clin Gastroenterol. 1982;4:321-4.
- [Google Scholar]
- Factors that can minimize bleeding complications after renal biopsy. Int Urol Nephrol. 2014;46:1969-75.
- [Google Scholar]
- Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev (2):CD008834.
- [Google Scholar]
- The effect of desmopressin acetate on postoperative hemorrhage in patients receiving aspirin therapy before coronary artery bypass operations. J Thorac Cardiovasc Surg. 1992;104:1417-22.
- [Google Scholar]
- Platelet transfusion for reversal of dual antiplatelet therapy in patients requiring urgent surgery: A pilot study. J Thromb Haemost. 2012;10:968-71.
- [Google Scholar]







