Translate this page into:
Optimization of Treatment Modality in Elderly End-stage Renal Disease Population: Peritoneal Dialysis versus Transplant
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Despite kidney transplantation (KT) being considered as the best treatment modality for end-stage renal disease (ESRD), patient and graft survival in the elderly population is poorer than younger individuals. Many authors argue that prolonged life expectancy outweighs the risk of remaining on dialysis, but few studies had compared the treatment modalities, especially with peritoneal dialysis (PD). A retrospective study was conducted at a tertiary care institute to compare outcome of elderly ESRD patients, who received KT with those continued on PD; and to evaluate the predictors of patient survival. Patient survival at 1 year was (76.2% vs. 91.1%); 5 years (53.7% vs. 21.8%); and 10 years (35.6% vs. 0.00%) among KT and PD population, respectively. Infection was the most common cause of death among KT group (35 [41.2%] vs. 34 [28.2%]) while cardiovascular mortality in PD group (55 [46.2%] vs. 7 [8.2%]). Technique survival at 1, 5, and 10 years in PD group was 92.8%, 58.5%, and 0%, respectively. Similarly, graft survival at 1, 5, and 10 years in KT group was 98.7%, 90.2%, and 90.2%, respectively. Multivariate analysis showed body mass index (BMI) (hazard ratio [HR] 0.88, 95% confidence interval [CI] 0.82–0.93, p < 0.001), and albumin (HR 0.55, 95% CI 0.37–0.80, p = 0.002) were significant predictors of survival. In the 1st year, patient survival was better in PD than KT, but after adjustment for BMI and albumin, both short-term and long-term survival in elderly KT group was better than that of PD. Hence, elderly ESRD patients should not be barred from KT just because of age.
Keywords
Dialysis
elderly
peritoneal dialysis
renal replacement therapy
renal transplant
Introduction
Chronic kidney disease (CKD) is a progressive disorder, which leads to end-stage renal disease (ESRD) and requires renal replacement therapy (RRT). With advances in treatment modalities aimed at slowing the disease progression, availability of better supportive care and improvement in life expectancy; ESRD is gradually becoming a geriatric condition.[123] Increase in the prevalence of diabetes and hypertension in the elderly population is also a risk factor for growing incidence of CKD-ESRD in this age group.[456]
While, there is no standard United Nations chronological age-based criterion, but it agreed on a cutoff of 60+ years to be referred as the older population.[7] It is associated with “Frailty,” “fall,” “functional,” and “cognitive” impairments; which are independent risk factors for mortality in elderly ESRD populations.[3] Uremia expedites all these issues in elderly patients.[38]
Hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT) are the established RRT options available for ESRD population. Due to economic, better patient survival, and improved mental health and quality of life (QoL), KT is considered as optimal modality of RRT.[91011] Moreover, studies on deciding optimal modality of RRT remain lacking and controversial in the elderly ESRD patient. Beyond geriatric syndrome, a number of other factors such as social support, financial assistance, and life expectancy are needed to be considered, while evaluating these patients for RRT. Although HD is a viable option, there are many adverse issues that the elderly face such as prolonged time to recovery, increased fistula failure rate, higher catheter-related tunnel infection and transport-related issues.[1213] There is also risk of intradialytic hypotension and myocardial stunning, for which rapid ultrafiltration is poorly tolerated.[13] Home-based therapy; slow and sustained ultrafiltration; and absence of vascular access-related issues make PD a better option.[13] However, it is limited by poor functioning, dementia, cognitive impairment, and inadequate social support.[1314] Due to age-related immunosenescence, elderly KT patients experience more infection-related complications than younger age group.[1516] Hence, while subjecting these patients for KT, immunosuppression should weigh against risk of infections.
In 2007, Rao et al. found that elderly KT recipients enjoy significant survival benefit over dialysis population.[17] However, due to a shortage of organs, one may argue against offering kidneys to the elderly as they have limited life expectancy and unable to enjoy a full life of the graft. For the same reason, organs from older donors are usually allocated to elderly recipients. Older kidneys are more immunogenic, thereby increasing the chance of rejection and the prescription of a more intense immunosuppression.[1819]
While a number of studies had compared “hemodialysis or predominantly HD” with KT, to the best of our knowledge, none had compared “only PD” with “KT.” Many authors argue that improved life expectancy; reduced risk of cardiovascular events, improved QoL, and better nutritional status can outweigh the risks of remaining on dialysis.[2021] Hence, we conducted a study at a tertiary care institute in the Northern India to compare the outcome of elderly ESRD patients 60 years and above, who received KT with those continued on PD; and hence, to evaluate the predictors of patient survival.
Subjects and Methods
Patient selection
This was a retrospective single-center observational study conducted at Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India between January 2001 and December 2015. It was conducted to compare outcome of elderly ESRD patients ≥60 years, who received KT with those continued on PD. Patients aged ≥60 years, who underwent KT or PD in above mention periods were included and followed until December 2016. Patient-related information such as age, gender, BMI, hemoglobin, albumin, exposure time, comorbidities, basic kidney disease, dialysis vintage, duration of hospitalization, frequency of infection-related hospitalization, and causes of death/graft loss/technique failure were collected from the hospital informatics system and hospital record section. Multiple renal allograft recipients, initiations of PD after graft failure and history of malignancy before start of RRT were excluded. This study was approved by the Institutional Ethics Committee and prior informed consents were obtained from all study subjects.
Exposure time was defined as the average time of exposure to either KT or PD and was expressed in per patient-year. Dialysis vintage for KT patients was defined as the duration of HD or PD before KT; and that for PD patients defined as the duration of HD before initiation of PD.
Outcome parameters
Patient survival was primary outcome of the study. Secondary outcomes were graft survival in KT recipients, technique survival in PD patients, duration of hospitalization, rate of infection-related hospitalization, anemia, and nutritional status. Patient survival was compared between groups. While calculating patient survival in KT recipients, death was the only event while other reason like transferred to dialysis was censored. Similarly, while calculating patient survival in PD group, death was the only event while transfer to HD, transplantation, or recovery of renal function was censored. Death occurring within 3 months of transfer was considered as event, while that occurring 3 months after transfer was censored.
Graft failure was defined as return to dialysis or GFR <10 ml/m2/min while death was censored. Technique failure was defined as permanent shift from PD to HD or KT due to inadequate dialysis, ultrafiltration failure (UFF), peritonitis, or exit site infection; while death, transplantation, recovery of renal function or shift to HD due to other causes were censored. Morbidity was monitored from the duration of hospitalization and rate of infection-related hospitalization. Nutrition status was monitored from body mass index (BMI), serum albumin, and hemoglobin.
Statistical analysis
Normality of the continuous variables was examined using Kolmogorov–Smirnov test. Data were presented in mean ± standard deviation for normally distributed variables and median (interquartile range “IQR”) for nonnormal variables. Unpaired Student t-test/Mann–Whitney U-test was used for comparative analysis between two groups. Pearson's Chi-square test/Fisher's exact test was employed to analyze categorical data as appropriate. Mortality was expressed both in percentage and per 100 patient-years (mortality rate). Kaplan–Meier method was used to calculate survival (patient and graft/technique) probability and comparison between the groups with corresponding significance level. Univariate and multivariate Cox proportional hazard model was used to evaluate predictors of patient survival. p < 0.05 was considered as statistically significant. Statistical package for social sciences, version-17 (SPSS-17, IBM Chicago, USA) was used for statistical analyses.
Results
Comparison of baseline characteristics among different group
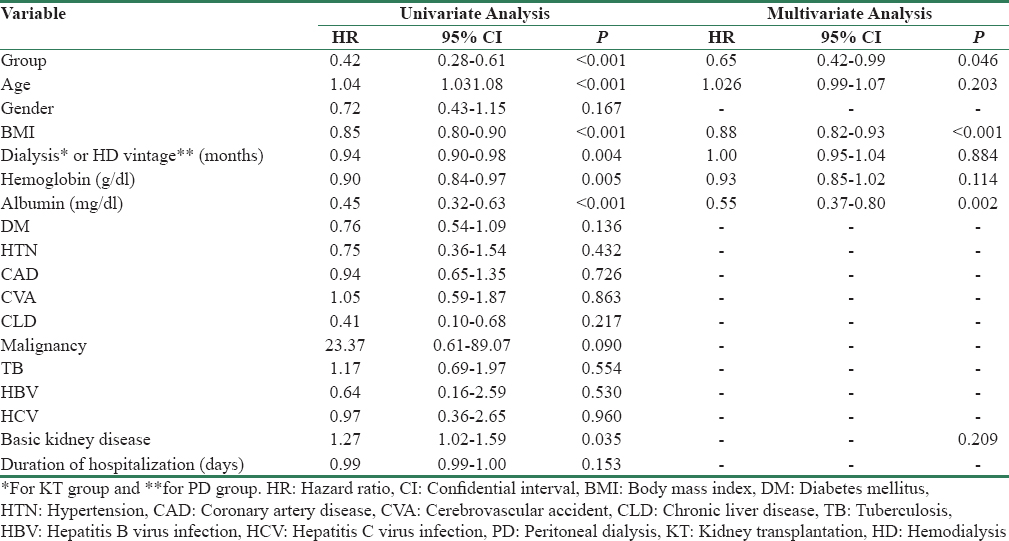
Comparison of baseline characteristics between the study groups has shown in Table 1. Totally 204 elderly ESRD were enrolled in the study, out of which 85 were KT recipients and 119 PD patients. All KT recipients had live-related donors. Out of 119 PD patients, only 8 patients were on automated PD. Median follow-up time for KT recipients was 24 (11–67.5) months (range 0.5-209 months) and that for PD patients was 21 (13–45) months (range 3-114 months). Exposure time in former was 4.03 patient-year while in later was 2.54 patient-year. Median age of KT group (61 [60–66] years) was lower than PD (69 [65–73] years) with p < 0.001. The percentage of female patients in PD (24 [20.2%])) was higher than KT (5 [4.7%], p = 0.002) group. Mean BMI of KT was slightly higher than PD patients (22.17 [±2.93] vs. 21.64 [±3.18] p = 0.227). Dialysis vintage was significantly higher in KT than HD vintage in PD group (8.00 [5.50–12.00] vs. 1.00 [1.00–2.00], months, p < 0.001). Minimum HD vintage for PD patients was 1 month in our study group. None of the patients was initiated on PD without HD. Hemoglobin and albumin were significantly higher in KT than PD group. There was no significant difference between the groups in regard to prevalence comorbidities such as hypertension, coronary artery disease, cerebrovascular accident, HCV, HBV, TB, and malignancy. The prevalence of DM was higher in PD (p = 0.035) whereas chronic liver disease was higher in KT group (p = 0.039). There was no significant difference in distribution of basic kidney disease as cause of ESRD among the groups (p = 0.119).

Factors affecting survival
Univariate Cox regression model was used to test the effect of covariates on patients’ survival. The result showed [Table 2] that variable, namely, group, age, BMI, dialysis vintage, hemoglobin, albumin, and basic kidney disease were significant independent predictors of survival. On multivariate analysis, we found that variable, i.e., BMI (HR 0.88, 95% CI 0.82–0.93, p < 0.001), and albumin (HR 0.55, 95% CI 0.37–0.80, p = 0.002) were the only significant predictors of survival.
Comparison of patients’ survival between the two groups
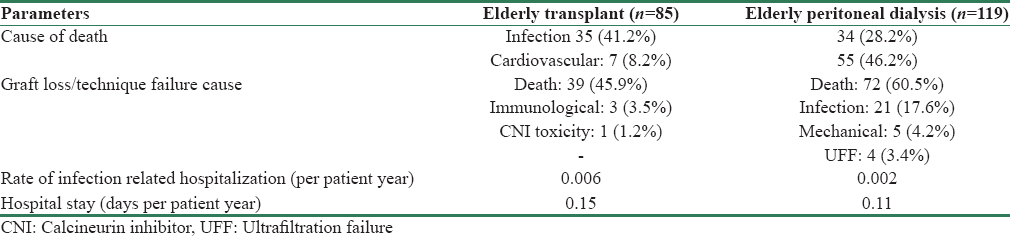
As depicted in Table 1, mortality rate was higher in PD group (29.24 vs. 12.31 per 100 patients-year). Overall mortality in KT group was 58% lower than elderly PD group (HR = 0.42, 95% CI = 0.28–0.61, p < 0.001). Comparison of patient survival between the groups is depicted in Table 3 and Figure 1. Overall survival was significantly lower in elderly PD group (p < 0.001). However, after adjustment for effect of significant predictors of survival such as BMI and albumin, mortality in KT group was 35% lower than PD group (HR = 0.65, 95% CI = 0.42–0.99, p = 0.046) improving both short- and long-term patients survival [Figure 2]. Infection was the major cause of death among KT population (35 [41.2%] vs. 34 [28.2%]), whereas cardiovascular-related disease in PD population (55 [46.2%] vs. 7 [8.2%]) [Table 4]. In PD group, 28.2% (n = 34) of mortality was due to infections; out of which 19.1% (n = 17) was related to peritonitis.
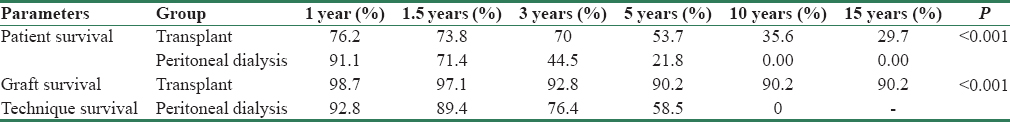
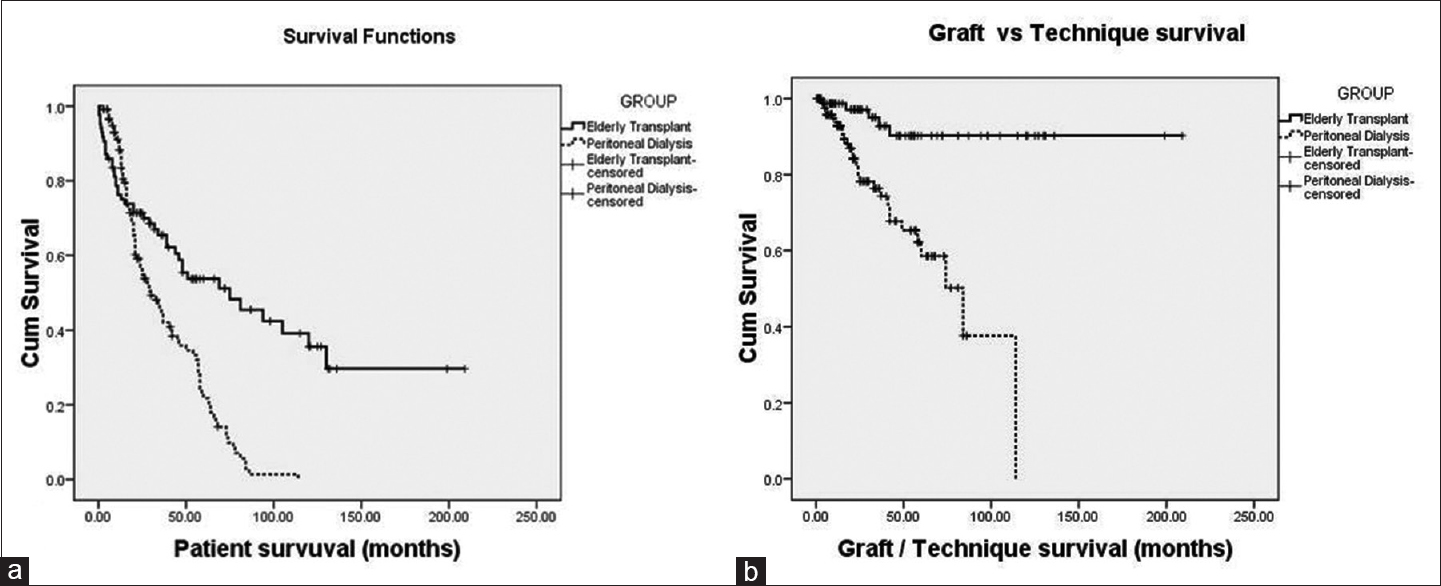
- Comparing survival analysis between elderly transplant recipients and elderly peritoneal dialysis. (a) Patient survival; (b) graft/technique survival
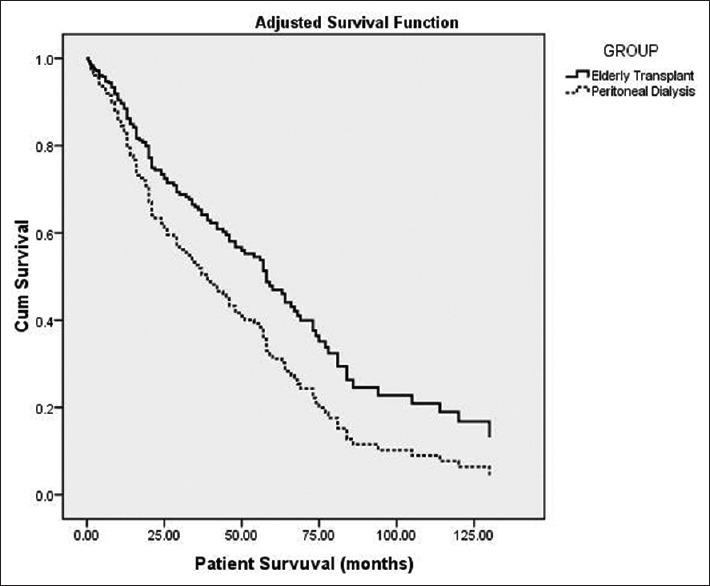
- Comparing adjusted survival analysis between elderly transplant recipients and elderly peritoneal dialysis
Assessment of graft and technique survival
Graft survival of KT recipients and technique survival of PD patients are depicted in Table 3 and Figure 1. Besides death (39 [45.9%]), the most common cause of graft loss was immunological (3 [3.5%]) followed by CNI toxicity (1 [1.2%]). Similarly, other than death (72 [60.5%]), the most common cause of technique failure was infection (21 [17.6%]) followed by mechanical (5 [4.2%]), and UFF (4 [3.4%]) [Table 4]. All infection-related technique failure was due to peritonitis only.
Comparison of morbidity between the two groups
Morbidity was monitored by the duration of hospitalization and rate of infection-related hospitalization. Rate of infection-related hospitalization was higher in KT than PD group (0.006 vs. 0.002 episode per patient-year). Similarly, hospital stay was also higher in KT than PD group (0.15 vs. 0.11 days per patient-year). There were total 55 episodes of peritonitis among the elderly PD patients with the rate of peritonitis being 0.29 episodes per patient-year. Most common etiology of peritonitis was culture negative (n = 32 [58.18%]), followed by Gram-negative (n = 12 [21.82%], Escherichia coli [7.27%], pseudomonas [7.27%]). Gram-positive, fungal and mycobacterial were accounted for 7.27%, 9.09%, and 3.63%, respectively.
Comparison of nutrition status between the two groups
Nutrition status was monitored from BMI, serum albumin and hemoglobin. BMI of KT was insignificantly higher than PD patients (22.17 [±2.93] vs. 21.64 [±3.18] p = 0.227), and hemoglobin and albumin were also significantly higher in KT than PD group with p ≤ 0.001. Multivariate analysis reveals that both BMI and albumin were significant predictors of patient survival.
Discussion
To the best of our knowledge, the present study is the only study comparing the outcomes among elderly patients who received KT to that with PD. The literatures till date have looked into outcomes comparing KT with that of dialysis populations predominantly consisting of HD patients.
In our study, patients enrolled in PD group were more elderly than KT group. This was due to reluctance by patients and family members for KT. Furthermore, the hospital policy to discourage transplant above 65 years of age, though not absolute, plays a significant role. The probable reason could be due to decreased life expectancy, compromised cardiovascular status, and increased risk of anesthesia-related complications. Age-related decreased immune functioning and tropical climate make them more prone to infections. Most elderly patients prefer homely environment than hospital-based services. However, many studies do not support this idea of age as a bar for renal transplant.[172022232425] Although PD was preferred modality of RRT for elderly patients in our institute, none of the patients underwent “PD First.” All patients were started on HD before initiation of PD, with minimum HD vintage of 1 month. This was due to poor acceptance among elderly patients for initiation of RRT. For the same reason, most patients were presented in late-stage with uremia-related complications needing urgent initiation of HD.
The unadjusted mortality rate in elderly KT group was lower than elderly PD group with the overall risk of death being 2.38 times lower. Long-term patient survival, i.e., 5 years and 10 years, in PD was also lower than KT group (p < 0.001). However, after adjusting for effect of significant predictors such as BMI and albumin, KT recipients had better, both short- and long-term survival, with risk of death 1.54 times lower compared to the latter group. Though till date none of the studies compared, elderly KT group with PD group, but studies involving predominately hemodialysis population had similar outcome.[17202223242526] In 1999, Wolf et al. concluded that cumulative survival improved in elderly KT group with survival benefit inversely proportional to age.[27] Availability of better immunosuppressant, improvement supportive care and careful patient selection might have improved both patient and graft survival in the current era.[20]
Comparing mortality in 1st year after initiation of RRT, we observed higher incidence mortality among KT, which is almost equalizes to PD population by 18 months and thereafter benefit improved with time. This was supported by Wolfe et al., Rao et al., and Heldal et al.[17202123] Wolfe et al. who reported that, in all age group, the risk of mortality during the first 2 weeks after transplantation was 2.8 times higher than patients on dialysis.[23] The reason could be due to the protocol designed to achieve the optimal immunosuppression during the intensive phase that is prone for rejection. Furthermore, treatment of acute rejection leads to over immune suppression and death in elderly.[2026] In our study also, we observed that infection was the most common cause of death in our KT group, justifying reason concluded by Heldal et al. also.[20]
Although 5 and 10 years, patient survival in our KT group was comparable to KT group of Rao et al., Similarly, survival of our elderly PD patients was comparable to that of elderly dialysis and PD patients of Heldal et al. and Sakacı et al. study group, respectively.[2029]
Cox univariate regression model in our study showed that age, BMI, albumin, hemoglobin, dialysis vintage, and basic kidney disease were predictors of survival. However, after application of multivariate regression, BMI, and albumin were the only significant predictors of survival. Unlike the study by Mazzuchi et al., our study did not support that diabetes, heart disease and cancer as significant predictors of survival.[25] After adjustment for BMI and albumin, we found that both short term and long term survival in elderly KT group was better than that of PD. This implicates that improvement in nutritional status, i.e., albumin and BMI, may improve both short-term and long-term survival in elderly KT group. As with other studies, our study also suggests that infection was the most common cause of death in elderly KT group, whereas cardiovascular in PD group.[25]
Technique survival in our PD population was significantly low (p < 0.001) compared to graft survival in KT populations. Technique survival is comparable to graft survival till 1½ years after which graft survival supersedes the technique survival. Most common cause of technique failure was infection followed by mechanical. All cases of infection-related technique failure were due to peritonitis only. It may be related to age-related immunosuppression that predisposes to increased rate and severity infection.
We used rate of infection-related hospitalization and total duration of hospitalization as markers of morbidity index. Both of these were lower in PD than KT group indicating that despite high mortality rate and relatively higher median age; morbidity was lower in former. This was supported by Meier-Kriesche et al. which state that exponential increase in infection and infection-related death in elderly KT recipients.[28] This could be due drug-induced immunosuppression as an important cause of infection and hospitalization in elderly KT group. However, Tonelli et al. in a systemic review, which reported that even elderly KT group has reduced risk of infection-related hospitalization.[21]
Peritonitis rate among PD group (0.29 episodes per patient-year) in the current study was comparable to other center; and even with the younger age group.[2729] Higher incidence of culture-negative peritonitis of the current study (58.18%) was supported by Prasad et al. (36.9%), Gupta et al. (50%) and a multicenter study from India by Abraham et al. (64.7%).[303132] Prasad et al. and Abraham et al. also supported the predominance Gram-negative over Gram-positive peritonitis of our study.[3032] A study by Kadambi et al. suggested that Gram-negative peritonitis was more prevalent among elderly.[33]
Besides being retrospective analysis and small sample size, the current study had few other limitations. Number of patients was more in elderly PD (n = 119) than KT (n = 85) group. Furthermore, median age of PD 69 (66–73) was higher than KT 61 (60–62) group. This is probably due to the following reasons – first, unwillingness by family members and patients for KT, expecting a shorter life expectancy, second, shortage of organs, third, institute protocol to discourage KT above 65 years of age although it was not an absolute contraindication, and later, is due to associated comorbidities and increased risk of anesthesia-related complications. However, after applying multivariate Cox regression analysis, we did not find age as a predictor of patient survival. Again, while calculating infection rate, we had only considered infections requiring admission as many patients received treatment at local centers for mild infections.
Conclusion
Although up to 1st year of initiation of RRT, patient survival is better in PD than KT; overall, long-term patient survival and nutrition status are better in renal transplant. Risk of cardiovascular events was significantly lower in KT group. As the magnitude of benefit improves with time, elderly should not be barred from KT. However, the absence of randomization, significantly higher age difference in the PD group and the retrospective analysis may be confounding factors to draw a definite conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- United states renal data system public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011). 2015;5:2-7.
- [Google Scholar]
- Maintaining safety in the dialysis facility. Clin J Am Soc Nephrol. 2015;10:688-95.
- [Google Scholar]
- Renal replacement therapy in the elderly population. Clin J Am Soc Nephrol. 2012;7:1039-46.
- [Google Scholar]
- Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26:1770-80.
- [Google Scholar]
- Changes in regenerative capacity through lifespan. Int J Mol Sci. 2015;16:25392-432.
- [Google Scholar]
- Proposed Working Definition of an Older Person in Africa for the MDS Project. Available from: http://www.who.int/healthinfo/survey/ageingdefnolder/en/
- [Google Scholar]
- Frailty and chronic kidney disease: The third national health and nutrition evaluation survey. Am J Med. 2009;122:664-7100.
- [Google Scholar]
- An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy. 2008;86:85-96.
- [Google Scholar]
- Health-related quality of life (HRQOL) in end stage renal disease (ESRD) patients over 65 years. Geriatr Nephrol Urol. 1998;8:85-94.
- [Google Scholar]
- Health related quality of life (HRQOL) of kidney transplanted patients: Variables that influence it. Clin Transplant. 2000;14:199-207.
- [Google Scholar]
- A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45:420-6.
- [Google Scholar]
- Peritoneal or hemodialysis for the frail elderly patient, the choice of 2 evils? Kidney Int. 2017;91:294-303.
- [Google Scholar]
- The elderly patient on CAPD: Helping patients cope with peritoneal dialysis. Perit Dial Int. 2008;28:449-51.
- [Google Scholar]
- The evolving notion of “senior” kidney transplant recipients. Clin Transplant. 2008;22:794-802.
- [Google Scholar]
- Renal transplantation in elderly patients older than 70 years of age: Results from the scientific registry of transplant recipients. Transplantation. 2007;83:1069-74.
- [Google Scholar]
- Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol. 2001;12:1538-46.
- [Google Scholar]
- Potent early immune response after kidney transplantation in patients of the European senior transplant program. Transplantation. 2009;87:992-1000.
- [Google Scholar]
- Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 2010;25:1680-7.
- [Google Scholar]
- Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093-109.
- [Google Scholar]
- How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945-51.
- [Google Scholar]
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- Superior outcomes of kidney transplantation compared with dialysis. Medicine (Baltimore). 2016;95:33.
- [Google Scholar]
- Comparison of survival for haemodialysis patients vs renal transplant recipients treated in uruguay. Nephrol Dial Transplant. 1999;14:2849-54.
- [Google Scholar]
- Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation. 2009;87:1045-51.
- [Google Scholar]
- Clinical outcomes and mortality in elderly peritoneal dialysis patients. Clinics (Sao Paulo). 2015;70:363-8.
- [Google Scholar]
- Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539-43.
- [Google Scholar]
- Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant. 2008;23:4021-8.
- [Google Scholar]
- Outcome of gram-positive and gram-negative peritonitis in patients on continuous ambulatory peritoneal dialysis: A single-center experience. Perit Dial Int. 2003;23(Suppl 2):S144-7.
- [Google Scholar]
- Epidemiology of culture isolates from peritoneal dialysis peritonitis patients in southern India using an automated blood culture system to culture peritoneal dialysate. Nephrology (carlton). 2011;16:63-7.
- [Google Scholar]
- Microbiology, clinical spectrum and outcome of peritonitis in patients undergoing peritoneal dialysis in India: Results from a multicentric, observational study. J Trop Dis Public Health. 2016;4:1-8.
- [Google Scholar]







