Translate this page into:
Hepatitis C Virus-associated Membranoproliferative Glomerulonephritis Treated with Directly Acting Antiviral Therapy
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hepatitis C virus (HCV) infection has been shown to affect kidneys with various histopathological pattern on the kidney biopsy. These commonly include a membranoproliferative glomerulonephritis (MPGN) pattern with mixed cryoglobulinemia (CG), thrombotic microangiopathy, membranous nephropathy, and vasculitis affecting medium and small vessels of the kidneys causing polyarteritis nodosa. It has been rarely associated with MPGN without detectable CG. We present one such patient who presented to us with HCV-associated MPGN without detectable CG, who recovered completely with directly acting antiviral therapy without any immunosuppression.
Keywords
Cryoglobulinemia
hepatitis C
membranoproliferative glomerulonephritis
Introduction
Hepatitis C virus (HCV) infection has been shown to affect kidneys with various histopathological patterns on the kidney biopsy.[1] These commonly include a membranoproliferative glomerulonephritis (MPGN) pattern with cryoglobulinemia (CG), thrombotic microangiopathy, membranous nephropathy, and vasculitis affecting medium, and small vessels of the kidneys causing polyarteritis nodosa (PAN).[2] We present one such patient who presented to us with HCV associated MPGN without detectable CG and managed with directly acting antiviral (DAA) alone.
Case Report
A 45-year-old male with a history of hypertension and chronic kidney disease (CKD) diagnosed concomitantly 2 years ago presented with acute onset rash [Figure 1] and diffuse joint pains for 2 weeks. His previous medical records revealed a baseline serum creatinine (S. Cr.) of 1.6–1.7 mg/dL. He denied any blood transfusions, intravenous drug abuse or high-risk sexual behavior. His medications included amlodipine and telmisartan. His blood pressure was 132/96 mm Hg, pulse rate 76/min and he had a diffuse erythematous maculopapular rash on the extensor surface of his lower extremities. His ankle joints were swollen, and movements were painful. He had 2+ bilateral pitting edema. His fundus examination was normal.
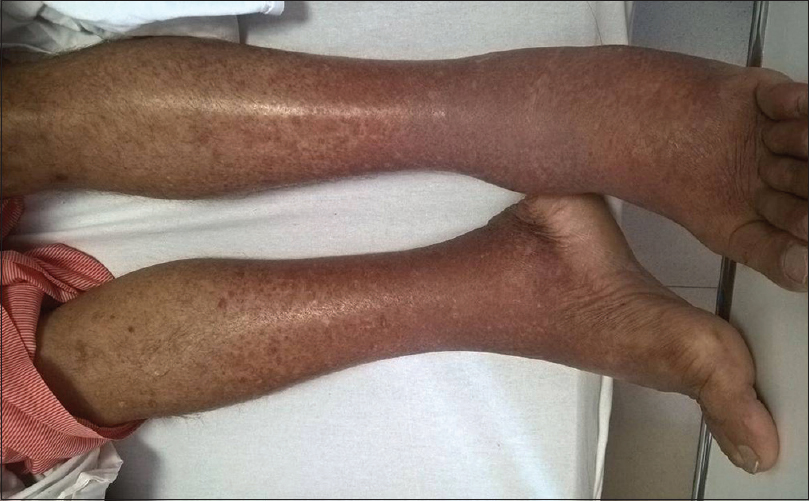
- Erythematous maculopapular rash on the extensor surface of lower extremities
Initial laboratory investigations revealed hemoglobin of 9.4 g/dL with a normal platelet and white cell count. His S. Cr. was 1.9 mg/dL and electrolytes were normal. His liver enzymes were normal and serum albumin was 2.9 g/dL. His initial urinalysis showed 3 + protein, 6–10 red blood cell/hpf and 4–6 white blood cell/hpf. His ESR was mm in 1st hour 38 and C-reactive protein was normal. An ultrasound revealed normal sized kidneys and chest X-ray was normal. His 24 h urine showed protein excretion of 3.1 g. His anti-nuclear antibody (ANA), HBsAg and HIV antibody tests were negative. Complements C3 and C4 were both low at 0.63 and 0.03 g/L, respectively. His anti-HCV antibody was positive with an HCV RNA viral load of 4133209 IU/ml and HCV genotype 3a. Complement cryoglobulin and cryocrit was undetectable.
An ultrasound-guided kidney biopsy was performed which showed enlarged glomeruli, with a global increase in mesangial matrix and cellularity, along with lobular accentuation. There was thickening of glomerular basement membranes and double contouring [Figure 2]. Few hyaline thrombi were seen within the capillary lumen. Interstitium showed mild edema with foci of chronic mononuclear inflammatory infiltrate. Immunofluorescence showed strong granular staining for C3, IgG and IgM along the capillary wall and in mesangial areas. Electron microscopy (EM) could not be done. A diagnosis of immune-complex mediated MPGN pattern was made.
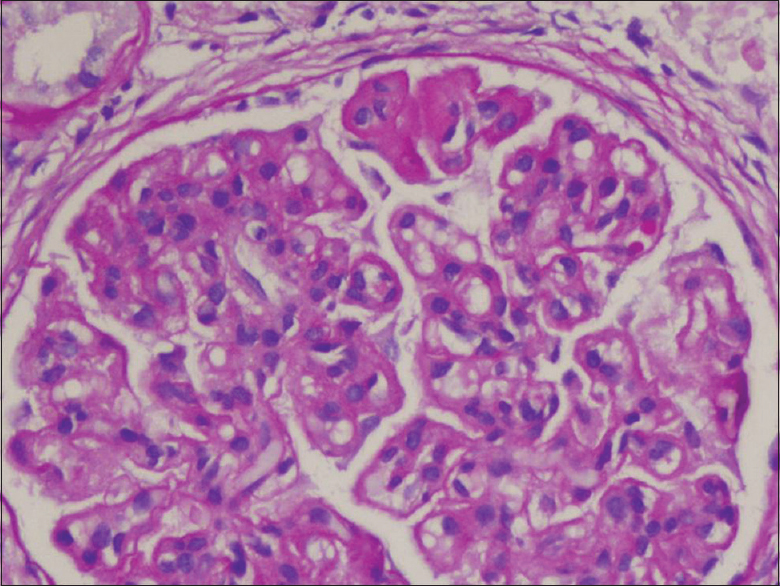
- Light microscopy (Hematoxylin and Eosin) showing enlarged hypercellular glomerulus with capillary wall thickening, double contouring and increased mesangial matrix and cellularity
The patient was started on DAA drugs-sofosbuvir 400 mg and daclatasvir 60 mg once daily for 12 weeks. Telmisartan was increased to 40 mg twice a day. A repeat HCV RNA test done after 6 weeks was undetectable. His S. Cr. gradually decreased to 1–1.2 mg/dL range with spot urine protein: creatinine ratio being 0.3–0.5 mg and continued to be stable at 12-month follow-up. Repeat quantitative HCV RNA testing done at 6 months and 1 year follow-up continues to be in the undetectable level.
Discussion
The prevalence of HCV infection in India is estimated to be as high as 5.2% in certain geographic area.[3] HCV infection has been shown to cause several distinct patterns of renal diseases such as MPGN, essential mixed CG, and membranous nephropathy.[12] Other less common pathologic findings in patients with HCV infection include PAN, focal segmental glomerulosclerosis, fibrillary, and immunotactoid glomerulopathies.[4]
It's clinical manifestations range from mild asymptomatic CKD[5] to frank nephrotic syndrome and finally end-stage renal disease.[6] It may even present as rapidly progressing renal failure owing to superimposed crescentic glomerulonephritis on any of the previously mentioned glomerular lesions.[6] Clinical features such as joint pains, skin rash, and low serum complement levels point toward an immune-complex-mediated phenomenon, which has been well known to happen in HCV infection.[7] Our patient had a preexisting mild CKD, likely from hypertension with an acute kidney injury likely secondary to immune complex-mediated glomerulonephritis secondary to HCV infection. HCV infection has been known to cause an MPGN like pattern on renal biopsy due to the deposition of HCV-immune complex deposits in the subendothelial region of the basement membrane.[8] This is commonly associated with systemic vasculitis owing to CG presenting clinically as a palpable purpura, arthralgias, peripheral neuropathy, retinal hemorrhage, and livedo reticularis.[8] HCV-associated MPGN usually is caused by CG Type II, although it may rarely be of type III CG.[9] The evaluation of patients for cryoglobulins requires meticulous specimen collection and processing. It involves the collection of blood samples in prewarmed tubes following which the serum is cooled to 4°C to allow the precipitation of CG and determination of a cryocrit.[10] However, in our case, CG were negative.
An EM examination of the kidney tissue is necessary to demonstrate the deposition of cryoglobulin along the glomerular basement membranes. HCV-associated MPGN without cryoglobulins in the blood has been previously reported in the literature. It may represent a true or a false negativity[10] and requires further confirmation of the diagnosis by light and EM. In a study by Prakash et al.,[11] none of the patients with HCV related MPGN had detectable CG.
The treatment of MPGN associated with HCV infection involves treatment of HCV.[12] With the advent of newer DAA, the treatment of HCV infection has been revolutionized and a complete eradication of the HCV infection is possible.[13] Treatment of these patients with newer DAA results in the remission of renal lesions and cryoglobulins in blood.[14] In addition to the treatment of HCV infection, these patients often also require the use of immunosuppression especially if they have a rapidly progressive renal failure, nephrotic range proteinuria or features of severe systemic vasculitis.[15] Immunosuppressive agents such as rituximab with or without plasmapheresis and corticosteroids have been shown to be effective in these patients.[1215]
Our patient improved rapidly with normalization of S. Cr. and improvement of proteinuria with DAA alone without requiring any immunosuppressive medication. This is likely due to the higher efficacy of new DAA's in eradicating the HCV and hence attenuating immune complex formation and further insult to the nephrons. He attained a rapid virologic response in <6 weeks, which remained sustained at a 1 year follow-up along with a stable renal function and minimal proteinuria.
Conclusion
HCV infection may cause MPGN without detectable CG. DAA therapy alone can cause complete recovery of the renal function and proteinuria in such patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his consent for his images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hepatitis C virus-related kidney disease: Various histological patterns. Clin Nephrol. 2010;74:446-56.
- [Google Scholar]
- Prevalence of hepatitis C virus in a selected geographical area of Northern India: A population based survey. Indian J Gastroenterol. 2012;31:232-6.
- [Google Scholar]
- Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998;9:2244-52.
- [Google Scholar]
- Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836-40.
- [Google Scholar]
- Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207-20.
- [Google Scholar]
- Systemic autoimmune diseases in patients with hepatitis C virus infection: Characterization of 1020 cases (The HISPAMEC registry) J Rheumatol. 2009;36:1442-8.
- [Google Scholar]
- Identification of glomerular immune deposits in cryoglobulinemia glomerulonephritis. Kidney Int. 1988;34:109-16.
- [Google Scholar]
- A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-5.
- [Google Scholar]
- Frequency and role of Hepatitis- C Virus and type II Cryoglobulinemia in Membranoproliferative glomerulonephritis. J Assoc Physicians India. 2004;52:451-3.
- [Google Scholar]
- Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev. 2011;10:444-54.
- [Google Scholar]
- Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-19.
- [Google Scholar]
- Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63:408-17.
- [Google Scholar]
- Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med. 2013;369:1035-45.
- [Google Scholar]







