Translate this page into:
Direct Correlation between Age at Diagnosis and Severity of Nephropathy in Fabry Disease Patients
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Nephropathy is one of the major complications of Fabry disease and mainly includes reduced glomerular filtration rate and proteinuria. Affected patients show different degrees of annual loss of renal function according to the magnitude of proteinuria and decrease in estimated glomerular filtration rate (eGFR) at the baseline.
Objetive:
To analyze the relationship between age at diagnosis and severity of nephropathy in a Fabry disease population.
Methods:
Cross-sectional design with retrospective data collection.
Results:
Seventy-two patients were studied with mean age of 26.26 ± 16.48 years and 30 men (41.6%). Twenty-seven paediatric patients and 45 adults were included. Thirteen genotypes were found: E398X, L415P, c886A>G, L106R, c.680G>A, A292T, c. 448.delG, R363H, C382Y, R301Q, D109G, del 3 and 4 exons, W81X, all pathogenic mutations of GLA gene. The mean eGFR in paediatric population was 115.81 ± 20.87 ml/min/1.73 m2 and in adults was 80.63 ± 42.22 ml/min/1.73 m2. The Pearson's bilateral correlation coefficient test (value = −0.462) between the age at diagnosis and eGFR indicates inverse correlation between both variables with a strong statistical significance (P = < 0.01). Spearman's bilateral correlation coefficient (value = +0.385) between the variables at diagnosis and the degree of proteinuria indicates direct correlation between both variables with a strong statistical significance (P = <0.01).
Conclusions:
Diagnosis of Fabry disease patients at a younger age could be a key to improve the nephropathy prognosis and allow early and effective interventions.
Keywords
Early diagnosis
estimated glomerular filtration rate
Fabry disease
nephropathy
proteinuria
Introduction
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by decreased or absent activity of lysosomal α-galactosidase-A (α-gal-A) activity, with progressive and multisystemic accumulation of globotriaosylceramide (Gb3) and its metabolites.[1] In the kidney, this accumulation is observed in glomerular cells, peritubular capillaries, vascular endothelial, smooth muscle cells, and tubular cells.[23] Progressive Gb3 accumulation is associated with life-threatening complications like renal failure, cardiovascular dysfunction, and stroke.[14] Nephropathy is one of the major complications of FD and mainly includes reduced glomerular filtration rate and proteinuria.[5]
Early diagnosis of nephropathy in FD patients is important. Prior to 2001, kidney disease was the major cause of death reported in affected patients.[6] Subsequently, it was described that life expectancy is decreased in FD patients of both sexes; the cardiovascular cause is the most frequent and patients who die by cardiovascular disease have two characteristics: (i) they previously received renal replacement therapy (RRT) for end-stage renal disease (ESRD) and, most importantly, (ii) they were diagnosed late.[7] In addition, affected patients show different degrees of annual loss of renal function according to the magnitude of proteinuria and the decrease in eGFR at the baseline.[8]
Enzyme replacement therapy (ERT) is a specific treatment for FD available since 2001. In Fabry nephropathy, its efficacy is greater the earlier it starts, a reduction of Gb3 tissue accumulation has been shown in a dose-dependent manner.[9] In more advanced stages of renal damage, its efficacy decreases due to the inability of correcting the progression when irreversible histological lesions are present, such as tissue fibrosis.[1011]
The aim of this study is to analyze the relationship between age at diagnosis and the severity of nephropathy in an FD population.
Subjects and Methods
In this retrospective study, patients with FD diagnosis were included from June 2007 to September 2017 from three reference centers in Argentina: (i) Los Manantiales Neuroscience Center, Grupo Gamma Rosario, Rosario, Santa Fe, Argentina; (ii) Center of Infusion and Study of Lysosomal Diseases of the Pergamino Clinical Nephrology Institute, Pergamino, Buenos Aires, Argentina; (iii) Intensive Unit Care of the Dr. Enrique Erill de Escobar Hospital, Belén de Escobar, Buenos Aires, Argentina. The diagnosis of FD was made by assessing enzyme activity α-gal-A [12] and molecular study.[13] Plasma and urine creatinine was determined by electro-chemiluminescence Roche Diagnostics. eGFR was calculated with Schwartz equation and CKD-EPI in patients under and over 18 years old, respectively.[14] To classify the eGFR stage The Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline 2013 (KDIGO) classification was used.[15] The albumin/creatinine ratio (ACR) in urine was used to estimate the urinary excretion of proteins in 24 h. Values from 0 to 30 mg/dl was considered normal, 30 to 300 mg/dl was indicative of albuminuria, and greater than 300 mg/dl indicated proteinuria, in at least two different samples of urine in all cases.
To estimate the degree of correlation between age and eFGR, the Pearson correlation coefficient was used. To estimate the degree of correlation between age and degree of proteinuria (ordinal with less than five categories), the Spearman correlation coefficient. It worked with a confidence interval (CI) of 95%. P values of <0.05 were considered of statistical significance to reject the null hypothesis.
The data were processed in IBM SPSS version 20 database. The study was approved by a local Ethics Committee. The adult patients signed an informed consent, and for the pediatric patientstheir legal representative gave consent according to local legislation.
Results
Seventy-two patients were studied with mean age of 26.26 ± 16.48 years and 30 men (41.6%). Twenty-seven pediatric patients and 45 adults were included. Thirteen genotypes were found: E398X, L415P, c886A>G, L106R, c.680G>A, A292T, c. 448.delG, R363H, C382Y, R301Q, D109G, del 3 and 4 exons, W81X, all pathogenic mutations of GLA gene.
The mean eGFR in paediatric population was 115.81 ± 20.87 ml/min/1.73 m2 and in adults was 80.63 ± 42.22 ml/min/1.73 m2. The mean ACR was 11.18 ± 11.10 mg/g and 386.63 ± 737.01 mg/g in paediatric patients and adults, respectively.
The FD complications frequency in studied population is shown in Table 1.
| Pediatric | Adults | |
|---|---|---|
| Gender (M/F) | 11/16 | 18/27 |
| Cornea verticillata (%) | 29.62% | 31.11% |
| Gastrointestinal discomfort (%) | 18.51% | 28.88% |
| Neuropathic pain (%) | 40.74% | 73.33% |
| Angiokeratomas (%) | 29.92% | 42.22% |
| Deafness (%) | 7.40% | 44.44% |
| LVH (%) | 0.00% | 44.445 |
| Arrhythmia (%) | 11.11% | 11.11% |
| CNS damage* (%) | 7.40% | 31.11% |
| Albuminuria/proteinuria (%) | 22.22% | 57.77% |
| eGFR decreased** (%) | 0.00% | 26.66% |
*Stroke and/or typical lesions in cerebral white substance in angionuclear magnetic resonance of brain. **eGFR <60 ml/min/m2. Ref: M: Males, F: Females, LVH: Left ventricular hypertrophy, CNS: Central nervous system, eGFR: Estimated glomerular filtration rate
Table 2 shows the inverse correlation between the age at diagnosis and eGFR in Pearson's coefficient test (value = −0.462; P = < 0.01), and Figure 1 shows the graphical representation of its linear correlation. Table 3 shows the direct correlation between age at diagnosis and degree of proteinuria in Spearman's coefficient (value = +0.385; P = <0.01).
| Age at diagnosis | eGFR | ||
|---|---|---|---|
| Age at diagnosis | Pearson correlation | 1 | −0.462** |
| Significance (bilateral) | 0.000 | ||
| n | 72 | 72 | |
| eGFR | Pearson correlation | −0.462** | 1 |
| Significance (bilateral) | 0.000 | ||
| n | 72 | 72 | |
**The correlation is significant at the 0.01 level (bilateral). eGFR: Glomerular filtration rate
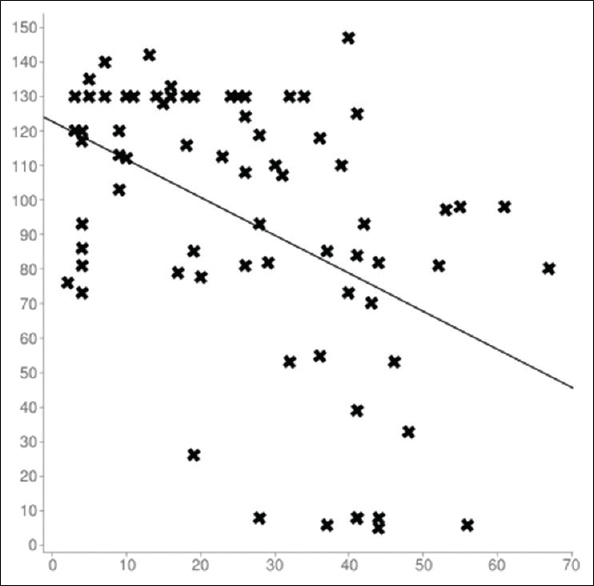
- Linear correlation between age at diagnosis and eGFRvariables. Sample size: 72; Mean x (x̄): 26.263888888889; Mean y (ȳ): 93.825; Intercept (a): 122.67968223109; Slope (b): −1.0986446962655; Regression line equation: y = 122.67968223109-1.0986446962655x. Ref: X axis: age (in years); Y axis: eGFR (in ml/min/m2)
| Age at diagnosis | Proteiuria | ||
|---|---|---|---|
| Rho of Spearman | |||
| Age at diagnosis | Correlation coefficient | 1.000 | 0.385** |
| Significance. (bilateral) | 0.001 | ||
| n | 72 | 72 | |
| Proteinuria | Correlation coefficient | 0.385** | 1.000 |
| Significance. (bilateral) | 0.001 | ||
| n | 72 | 72 |
**The correlation is significant at the 0.01 level (bilateral)
Discussion and Conclusions
Nephropathy is a major complication of FD patients,[15] with a related increased morbidity and mortality.[7] Kidney damage can begin at a very young age,[16] and glomerular-sclerosis and vascular lesions have been described in renal biopsies of children and adolescents who had not yet exhibited decreased eGFR or overt proteinuria.[29171819]
In the natural course of Fabry nephropathy, the annual decline in renal function over time is related to the degree of proteinuria, male sex, and is faster when the initial eGFR is less than 60 ml/min/1.73 m2.[48] Progression of renal damage culminates in ESRD. Affected males with “classical” mutations of the GLA gene, which produces severe decrease in α-gal-A activity, require RRT for ESRD treatment at ages of 35–44 years.[20] Therefore, the severity of the nephropathy conditions the evolution of FD patients, and the search for factors of poor renal prognosis is useful in affected patients.
Early biomarkers of prealbuminuric-stage nephropathy have been studied in FD patients with promising results,[2122] because it is known that urinary protein excretion is present when the renal tissue lesions are irreversible.[2916171819] The purpose of these investigations is to detect early renal damage before irreversible structural damage occurs in renal tissue.
The prototype of irreversible renal damage is renal fibrosis, both in glomerulosclerosis or tubulo-interstitial fibrosis.[10] Renal fibrosis, characterized by excessive deposition of extracellular matrix (ECM), is recognized as a common pathological feature of chronic kidney diseases (CKD), which is in direct relation to progression and leads to the development of ESRD.[23] As in other causes of nephropathy, there are no effective treatments that can reverse renal fibrosis in FD. In our study population, a direct relationship was found between age at diagnosis of FD and the severity of the nephropathy, as determined by eGFR and the degree of proteinuria.
The Pearson's bilateral correlation coefficient test (value = −0.462) between the age at diagnosis and eGFR indicates inverse correlation between both variables with a strong statistical significance (P = <0.01). At higher age, patients present with lower eGFR. On the other hand, the Spearman's bilateral correlation coefficient (value = +0.385) between the age at diagnosis and the degree of proteinuria indicates direct correlation with a strong statistical significance (P = <0.01). At higher age at diagnosis, patients have higher proteinuria.
ERT is more effective the earlier it is started; in a dose-dependent manner it is able to revert tissue lesions, even in injured podocytes.[9] In more advanced stages of nephropathy, ERT is less effective because it is unable to reverse renal fibrosis.[9102425]
In conclusion, the diagnosis of FD at a younger age could be a key to improve the prognosis of nephropathy and allow early and effective interventions.
Financial support and sponsorship
SJ have received speaker fees from Genzyme, SHIRE and Biomarin; and and support for research work. NA received speaker fees from Genzyme and Shire and travel grants from Genzyme, Shire and Protalix. FP have received speaker fees from Genzyme.
Conflicts of interest
There are no conflicts of interest.
References
- Scoring system for renal pathology in Fabry disease: Report of the International Study Group of Fabry Nephropathy (ISGFN) Nephrol Dial Transplant. 2010;25:2168-77.
- [Google Scholar]
- Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933-46.
- [Google Scholar]
- Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24:2102-11.
- [Google Scholar]
- Prevalence of chronic kidney disease in fabry disease patients: Multicenter cross sectional study in Argentina. Mol Gen Metab Rep. 2017;12:41-3.
- [Google Scholar]
- Fabry Disease. Perspectives from 5 Years of FOS. Oxford PharmaGenesis 2006 Chapter 19
- [Google Scholar]
- Life expectancy and cause of death in males and females with Fabry disease: Findings from the Fabry Registry. Genet Med. 2009;11:790-6.
- [Google Scholar]
- Prognostic indicators of renal disease progression in adults with fabry disease: Natural history data from the Fabry registry. Clin J Am Soc Nephrol. 2010;5:2220-8.
- [Google Scholar]
- Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24:137-48.
- [Google Scholar]
- Fibrosis: A key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8:116.
- [Google Scholar]
- Time to treatment benefit for adult patients with Fabry disease receiving agalsidase β: Data from the Fabry registry. J Med Genet. 2016;53:495-502.
- [Google Scholar]
- Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785-96.
- [Google Scholar]
- Identification of GLA gene deletions in Fabry patients by Multiplex Ligation-dependent Probe Amplification (MLPA) Mol Genet Metab. 2008;94:382-5.
- [Google Scholar]
- Monitoring renal function in children with Fabry disease: Comparisons of measured and creatinine-based estimated glomerular filtration rate. Nephrol Dial Transplant. 2010;25:1507-13.
- [Google Scholar]
- Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Inter Med. 2013;158:825-30.
- [Google Scholar]
- Progressive podocyte injury and globotriaosylceramide accumulation in young patients with Fabry disease. Kidney Int. 2011;79:663-70.
- [Google Scholar]
- Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron. 2015;129:16-21.
- [Google Scholar]
- Early renal involvement in a girl with classic Fabry disease. Case Rep Nephrol. 2017;9543079 doi: 10.1155/2017/9543079
- [Google Scholar]
- Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767-76.
- [Google Scholar]
- Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61:249-55.
- [Google Scholar]
- Increased urinary CD80 excretion and podocyturia in Fabry disease. J Transl Med. 2016;14:289.
- [Google Scholar]
- New biomarkers defining a novel early stage of Fabry nephropathy: A diagnostic test study. Mol Genet Metab. 2017;121:162-9.
- [Google Scholar]
- Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med. 2007;146:77-86.
- [Google Scholar]
- Treatment of Fabry's disease with the pharmacologic chaperone Migalastat. N Engl J Med. 2016;375:545-55.
- [Google Scholar]







